
Status
Research scientist at CEA DSM/IRAMIS/NIMBE/LIONS, Expert CEA
Research Topics
Gold nanoparticles (mechanism of formation) / Microemulsions (structure-reactivity in complex fluids)
Gold nanoparticules: mechanism of formation, control of shape, size and internal structure

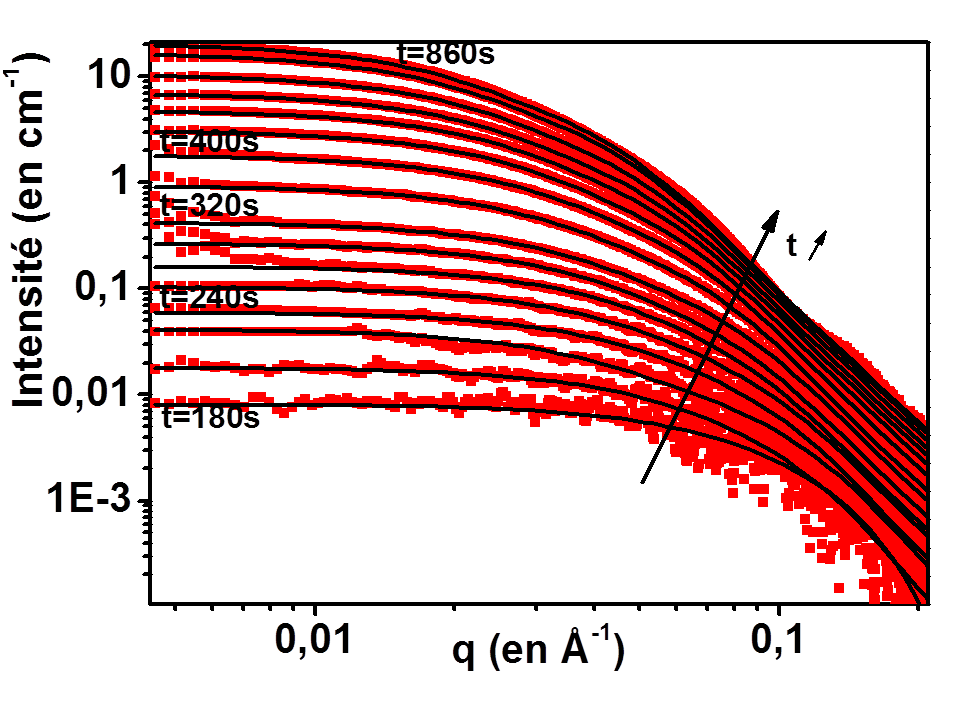
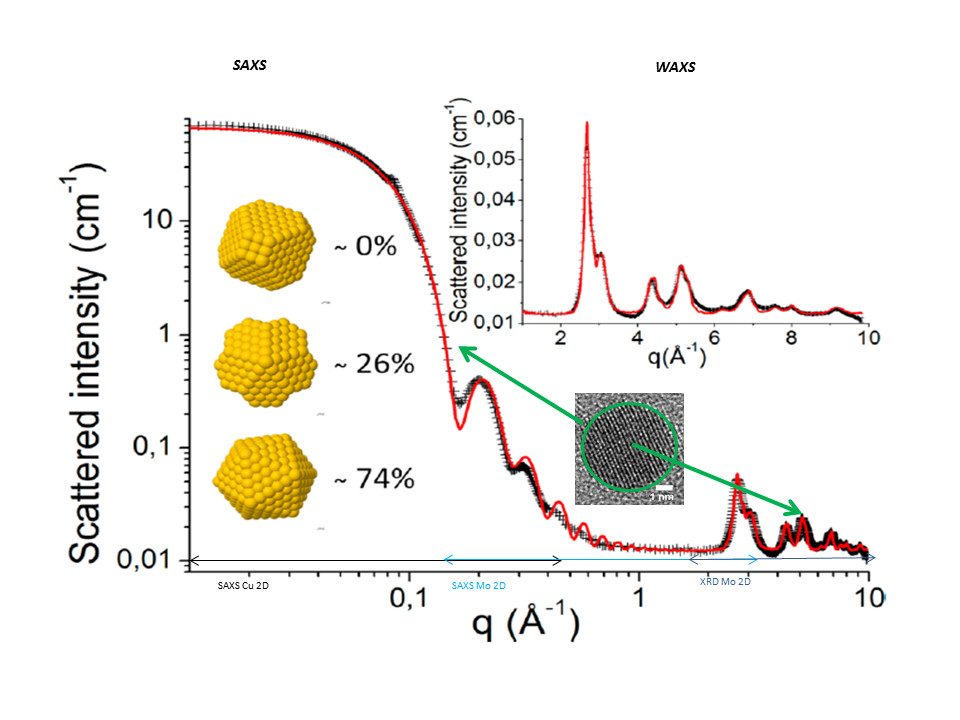
Since 2006, we developed methodologies for in situ kinetic studies on the formation of gold nanoparticles to attain the dynamic phenomenon in nanomaterials with an extraordinary time resolution (by taking benefit of 3rd generation synchrotron). While several synthetic pathways for gold nanoparticles have been developped in the community, there is still a need for a better understanding of the mechanism of formation and development of complete characterizations methods. If size control is nowadays achieved, the control of the shape and internal structure still remains a difficult point.
For spherical nanoparticles, we evidenced the key role of the injection law of monomers. For anisotropic shapes obtained by seeded growth method, we evidenced the role of the initial structure of seeds and ratio of growth rate in different direction to control the final shape. The originality of our approach comes from: (i) the acquisition of original data focusing on the occurrence of anisotropy (ii) a strong coupling between experiments and theoretical simulations.
Beyond the control over size and shape, the organisation and incorporation into an organic framework is indispensable to modulate the collective properties. We start to developp incorporation of nanoparticles into 2D organized diblock copolymers to go towards metamaterials.
- “Gold Nanoparticle Internal Structure and Symmetry Probed by Unified Small-Angle X‑ray Scattering and X‑ray Diffraction Coupled with Molecular Dynamics Analysis”
B. Fleury, R. Cortes-Huerto, O. Taché, F. Testard, N. Menguy, and O. Spalla, Nanoletters (2015), 15, 6088−6094
(2nd POSTER PRICE ADD 2016 – Analysis of Diffraction Data) - « Twinned Gold Nanoparticles under Growth: Bipyramids Shape Controlled by Environment”
Z. C. Z. Canbek, R. Cortes-Huerto, F. Testard, O. Spalla, S. Moldovan, O. Ersen, A. Wisnet, G.Wang, J. Goniakowski, C. Noguera, and N. Menguy, Cryst. Growth Des. (2015), 15, 3637−3644. - « Understanding of the Size Control of Biocompatible Gold Nanoparticles in Millifluidic Channels »,
J. Han, F. Testard, F. Malloggi, P-E. Coulon , N. Menguy , O. Spalla, Langmuir, (2012), 28 15966−15974.
Post-doctorant: B. Fleury (2013-2014)
PhD codirection: B. Abécassis (2006, Ecole Polytechnique), F. Hubert (2009, UPMC), Han Jun (2012, UPMC), Z. C. Canbek (2014, UVSQ)
Coordinator MIGRANI ANR project (2011-2014): ANR11 BS10 006 01 based on an interdisciplinary consortium of four laboratories (LIONS (CEA, CNRS), INSP (CNRS, Paris VI), IMPMC (CNRS, Paris VI), PHENIX (CNRS, Paris VI)))
Multifonctionnal microemulsions-reactivity in complex fluid
In the field of microemulsions, there is a huge interest to go towards multifunctional properties for new materials and new applications. The versatility of their structures (droplets to bicontinuous) and their high solubilization power can be merged with added properties coming from the use of a specific polar or apolar phases or from multifunctional surfactants. We developed in our laboratory, a field of research on the relation between structure and reactivity in these new complex solutions.
Nanoprecipitation-nanodrugs
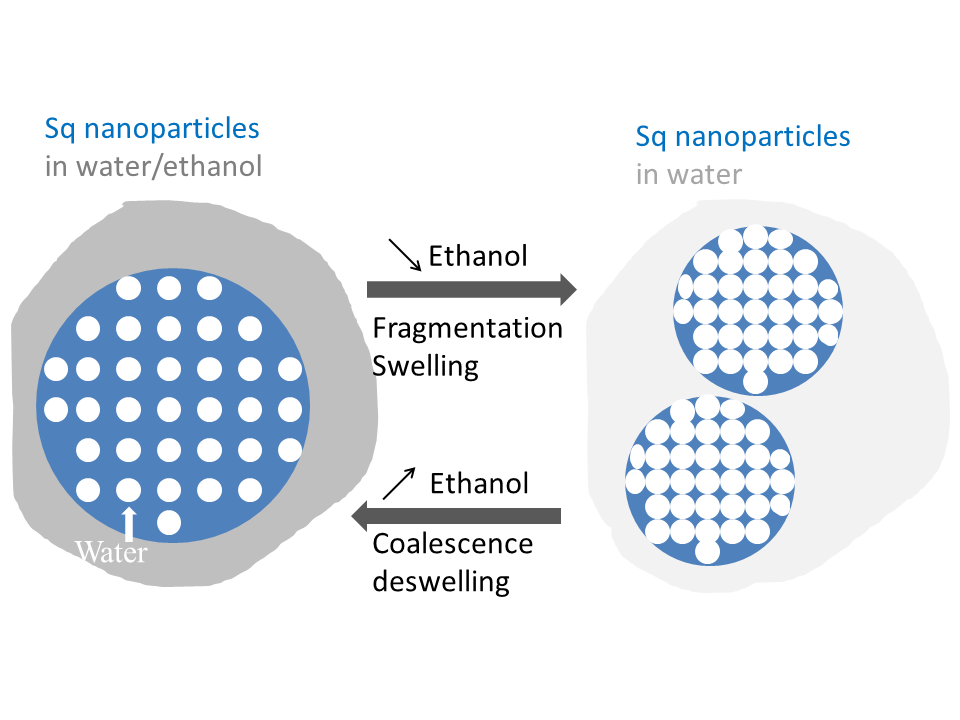
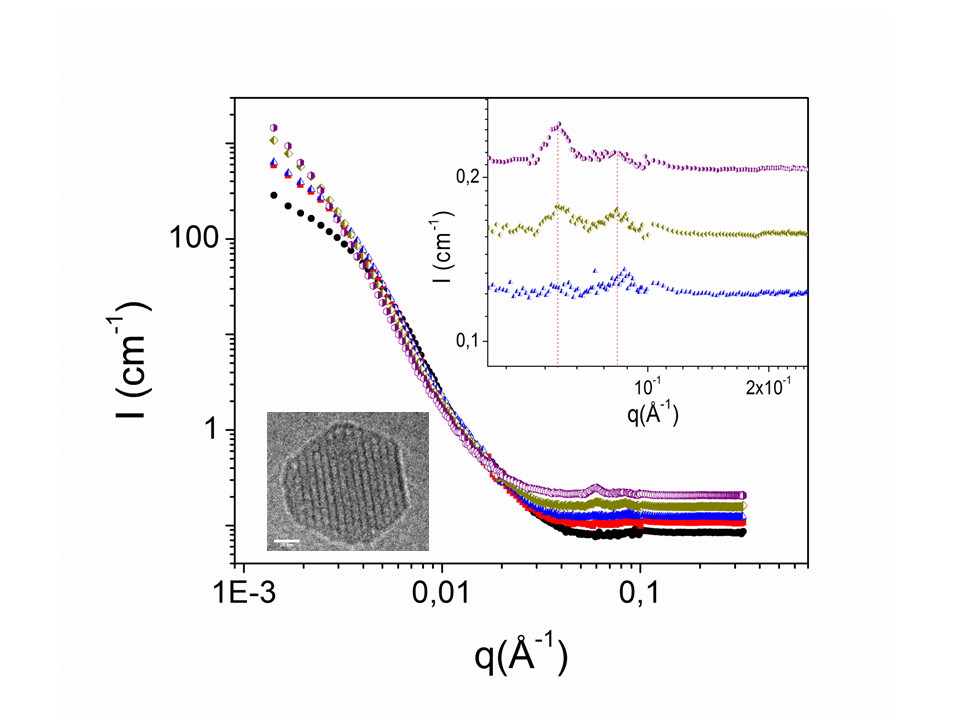
In the recent years, it has been proved that gathering in one molecule self-assembling properties with pharmaceutical function can enhanced the therapeutic activities. In the 2006’s, P. Couvreur (Université Chatenay-Malabry) has introduced the squalenoylation technology to develop new nanodrugs with improved efficiency (Couvreur et al, Nanoletter (2006, 6,2544)). In 2012, in collaboration with this team, we started to investigate the mechanism of formation of these particular nanodrugs obtained by the covalently link between different nucleoside analogues (known for their anticancer or antiviral properties) to the acyclic isoprenoid chain of squalene and formulated into nanoparticles trough nanoprecipitation processes. The final aim is the control in size distribution, internal structure and stability in biological media.
Dedicated to the particular case of nanodrugs, this research activity deals with the famous “ouzo” region and “surfactant free” microemulsion.
Recently, in the framework of the project « Nanoprotection » (Labex NanoSaclay, flagship 2), we start a study on the structure and the future of the nanoparticles of Squalene-adenosine ( SqAd) in biological environment. NEW!
- « The role of solvent swelling in the self-assembly of squalene based nanomedicines »
D. Saha, F. Testard, I. Grillo, F. Zouhiri, D. Desmaele, A. Radulescu, S. Desert, A. Brulet, P. Couvreur and O. Spalla; Soft Matter (2015) 1 4173-4179.
Postdoctorant : D. Saha (2012-2014), J. Bizeau (Master Internship 2017)
PhD: E. Marret (2015-2018) financed by ILL and ECE, paris
Financement: Labex Nanosaclay ANR-10-LABX-0035,
Reactivity in emulsion
Emulsion precipitation can be implemented on a large scale through the use of pulse columns which disperse large quantities of aqueous phase in the form of droplets in an organic phase. Precipitation occurs after coalescence of two drops each containing two different reactants. If the feasibility of such a process is now proved (use in the field of nuclear waste treatment), partial knowledge of the reaction mechanism is a limiting step. We propose in this project to study this process by using controlled systems. This project is supported by a collaboration between two CEA laboratories: the SA2I in Marcoule ( contact Sophie Charton) and the LIONS in Saclay (contacts : Antoine Thill and Fabienne Testard).
Beyond studies on the coalescence between reactive droplets, we investigate the feeding of reactant through the organic continuous phase. Fisrt studies focuses on grafted imogolites with the phD ( EDI, Univ. Paris Saclay) of Tobias Lange (start in Oct. 2016) NEW!
PhD co-direction : T. Lange (2016-…,)
Liquid/liquid extraction
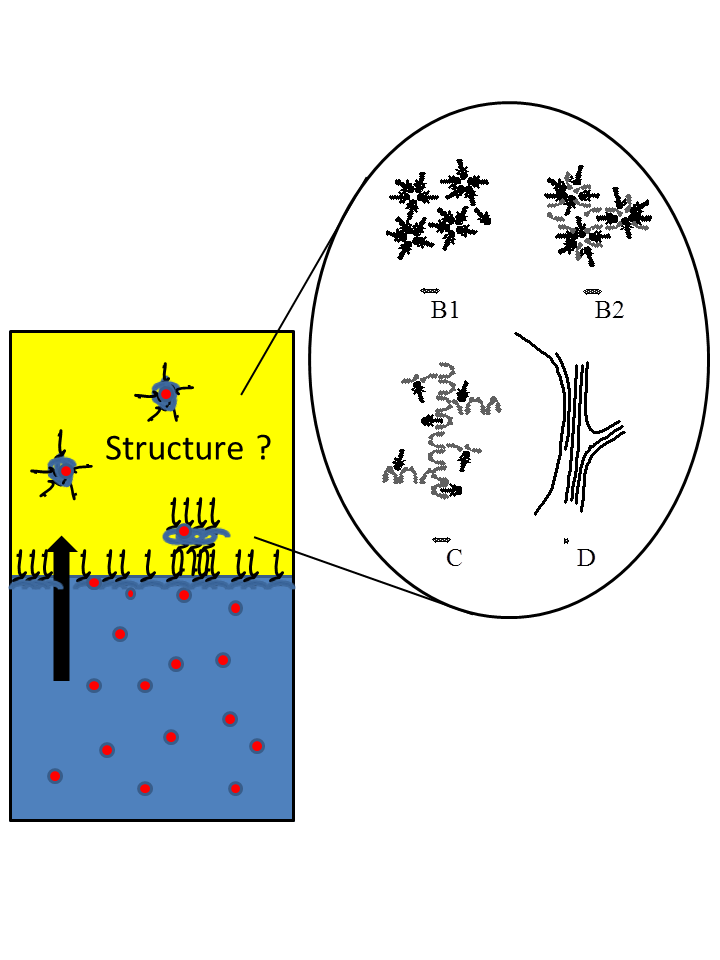
Multifonctionnal microemulsions have been largely used in the field of liquid/liquid extraction where extractant can play the dual role of surfactant and chelating agent. The so-called organic phase can be described as a microemulsion with chelating properties able to extract selectively some ions from an aqueous phase. In collaboration with CEA/DEN and CEA/ICSM, the structure of these particular extractant organic phases has been studied and rationalized through the properties of the curvature of the interfacial film between polar and apolar phases. Beyond the relation between structure and extraction efficiency, recent development in the understanding of synergism for a combination of extractants have been obtained. We also demonstrate the feasibility of kinetic measurement across the oil/water interface by the use of microfluidic chips.
- » Uranium extraction by a bifunctionnal amido-phosphonic acid: coordination structure and aggregation »
O. Pecheur; D. Guillaumont; S. Dourdain, L. Berthon , R. Turgis, C. Fillaux, G. Arrachart, F. Testard, Solv. extr. ion Exch. (2016), 34, 260-273. - « Synergism in a HDEHP/TOPO Liquid−Liquid Extraction System: An Intrinsic Ligands Property ? »
O. Pecheur; S. Dourdain, D. Guillaumont, J. Rey, P. Guilbaud, L. Berthon, M.C. Charbonnel, S. Pellet-Rostaing, F. Testard J. Phys. Chem. B (2016), 120, 2814- 2823. - “Synergism by Coassembly at the Origin of Ion Selectivity in Liquid-Liquid Extraction”
Dourdain, S; Hofmeister, I; Pecheur, O; Dufreche, JF; Turgis, R; Leydier, A; Jestin, J; Testard, F; Pellet-Rostaing, S; Zemb, T Langmuir (2012), 28 11319-11328
PhD co-direction : O. Pecheur (2014, UVSQ)
Postdoctorant : S. Nave (2002-2004), P. Bauduin (2005-2007)
European Network: n° PARTNEW-FIKW-CT2000-00087 (2000-2003); n° EUROPART-FP6-508854 (2004-2007)I
Solid microemulsion – long chain fatty acid
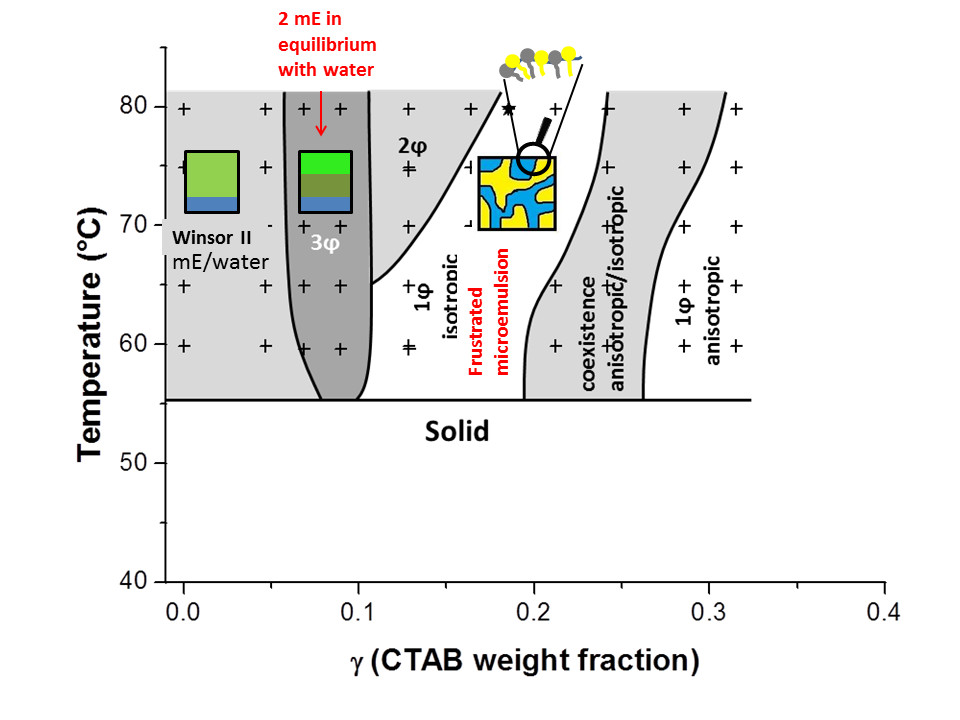
Going towards solid microemulsion is very promising to keep bicontinuous structure and high specific surface within a solid material. Recently, with D. Carriere (CEA/LIONS), we take advantage of the solvosurfactant property of long chain fatty acid above their melting point to investigate the formation of microemulsions with a ternary system water/ cationic surfactant (cetyltrimethyl ammonium bromide) and myristic acid. The existence of a frustrated microemulsion driven by the association of fatty acid with CTAB was demonstrated, while temperature driven solidification fails by the segregation of the MA-CTAB surfactant film from liquid MA. Initially developed for materials with proton conducting, such approach with fatty acid solvosurfactant reveals an original behavior with promising towards new formulations.
- « Nanostructure changes upon polymerization of aqueous and organic phases in organized mixtures »
C. Noirjean; C. Vancaeyzeele, S. Bourcier, F. Testard, F. Vidal, D. Carriere; O. Fichet; Langmuir (2016), 32, 10104-10112 - “Molten fatty acid based microemulsions”
C. Noirjean; F. Testard; C. Dejugnat; J. Jestin; D. Carriere; Phys. Chem. Chem. phys. (2016), 18, 15911-15918. - “Quenched microemulsions: a new route to proton conductors”
C. Noirjean; F. Testard; J. Jestin; O. Tache; C. Dejugnat; D. Carriere; Soft Matter (2014), 10, 5928-5935.
PhD codirection: Noirjean (2014, UVSQ)
Functionnalised surfactants for extraction or catalysis:
In collaboration with UVSQ (Ch. Larpent, Institut Lavoisier, UVSQ), new thermoresponsive chelating surfactants were developed with high promising towards new solvent free separation processes. A real molecular economy was demonstrated in cloud point extraction of uranyl nitrate in comparison to classical liquid-liquid extraction or classical cloud point extraction only based on solubilisation properties. This class of functional surfactants with organocatalyst functions is also powerfull to catalyse organic reactions in aqueous phases.
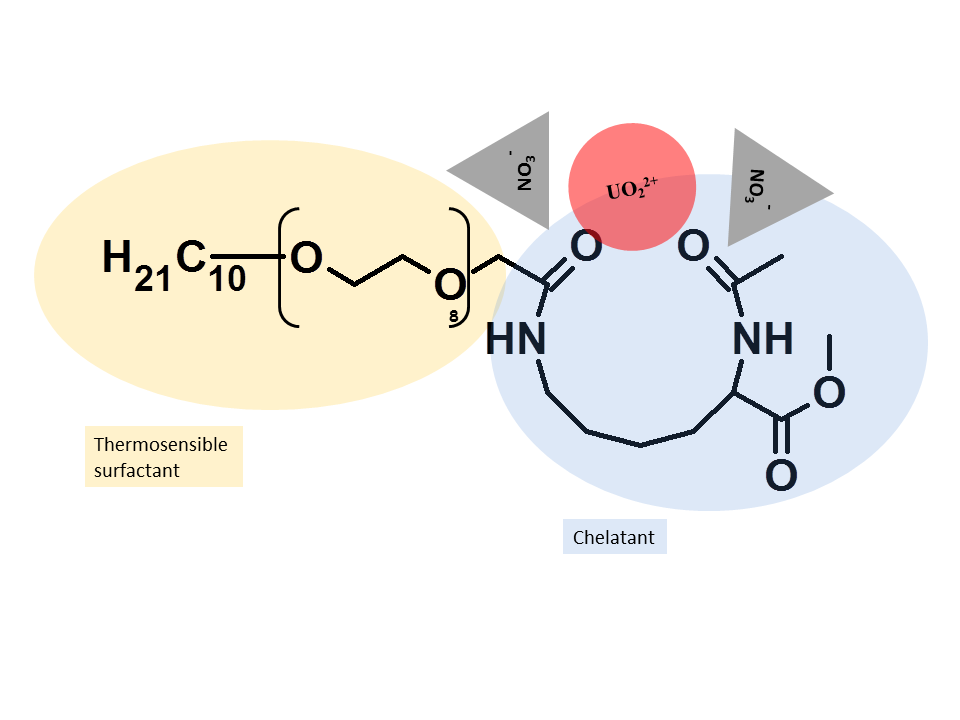
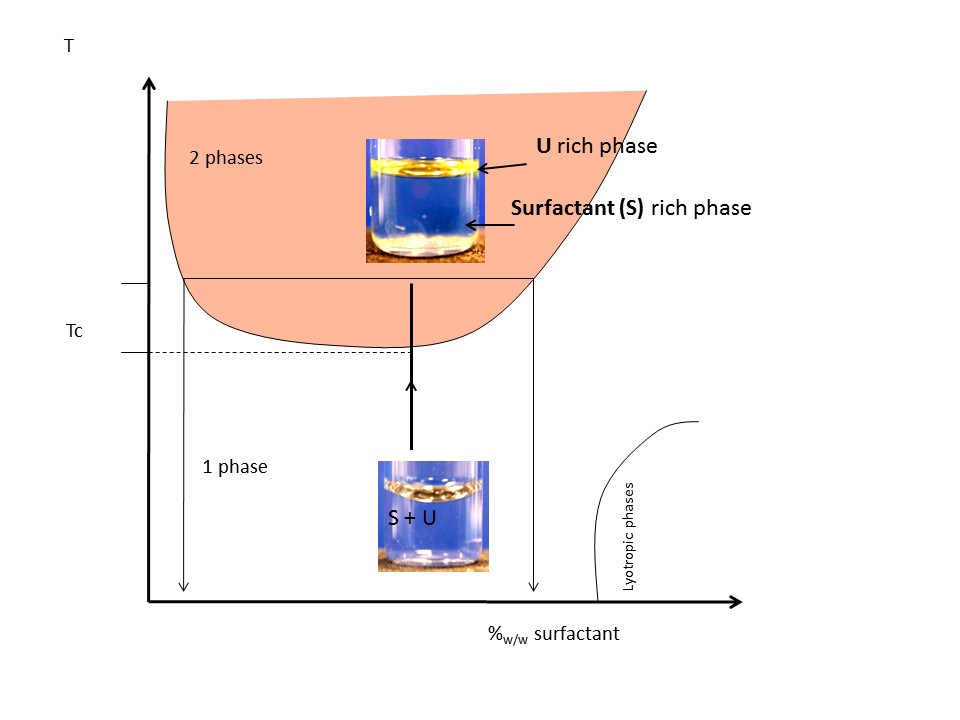
- ” Nonionic Metal-Chelating Surfactants Mediated Solvent-Free Thermo-induced Separation of Uranyl”
Larpent C., Prévost S., Berthon L., Zemb Th.,and Testard F., New Journal of Chemistry (2007), 31, 1424-1428. - Patent: « Composé thermosensibles présentant un point de trouble, utilisables comme extractants pour la séparation de métaux en solution aqueuse”
Inventeurs: Ch. Madic, L., Berthon, N., Zorz, H. Coulombeau, F., Testard, Th., Zemb, S., Gibanel, Ch., Larpent, K., Baczko, Eur. Patent EP 1425363 (2004)
PHD co-direction: H. Coulombeau (2003, UVSQ), S. Prévost (2006, UVSQ), R. Gadou (2010, UVSQ)
Post-doctorant: S. Lassiaz (2006-2007)
CV
| 1997- Today | Research Scientist at CEA/DSM/IRAMIS |
| 2009 | HDR (Université de Versailles St Quentin en Yvelines) « De la molécule aux propriétés macroscopiques d’une solution complexe : relation structure-réactivité de solutions complexes et nucléation de nanoparticules » |
| 1996-1997 | PRAG position at Université Paris V |
| 1993-1996 | PHD (Université Pierre et Marie Curie, Paris VI) « Solubilisation de molécules hôtes dans un film de tensioactif : approche thermodynamique et structurale dans le cas du lindane » |
| 1992 | Agrégation de physique Option-chimie |
| 1989-1993 | ENS-Lyon |
Teachings
Lectures:
| 2014 | « Chemical functionality and weak interactions : Syntheses, Assemblies, Materials : ‘Synthesis of nanoparticles: improving understanding of size and shape control with kinetic studies” LABEX CheMiSyst” summerschool (St. Martin de Londres, France) (1H) |
| 2011 | « Introduction to Colloïdes and nanoparticules: stability, self assembling, microemulsions and other aggregates, solubilisation », ICSM, « separative chemistry » (3H) |
| 2010 | « Structure of colloïdal solution, small angle X-rays/neutrons scattering » Master M2 CIF (Lille) (3H) |
| 2007 | « Colloïdal approach in liquid/liquid extraction » ICSM summer school (Bagnols sur cèze) (1H) |
| 2005-2011 | Coordination in CEA for the students from the European Research Master : Colloïdal and supramolecular chemistry (UVSQ, University of Regensburg, University of Florence) |
| 2005-2006 | « Milieux dispersés, surface et interface » master2 (UVSQ) (20H) |
| 2004-2006 | « Physical chemistry f dispersed systems » European fragance and cosmetic master ISIPCA (9H/year) |
| 2004 | « Micellar stability and supramolecular approach of liquid/liquid extraction” Actinet summer school (Avignon) (1H) |
| 2003-2004 | « Milieux dispersés, surface et interface » DEA M3RI (UVSQ) (14H) |
| 1996-1997 | PRAG position (Paris V) 394H /an (TP, TD, Organic chemistry, thermodynamic, …) |
| 1994-2003 | TD « Experimental methods for small angle scattering »in DEA « Matière condensé, chimie et organisation » (8H/year) |
Lectures in summer school:
- Lecture to summer school LABEX CheMiSyst” (St. Martin de Londres, France) Sept 14 :
« Synthesis of nanoparticles: improving understanding of size and shape control with kinetic studies” F. Testard. - Lecture to Actinet summer school (Avignon, France) June 04 : “Micellar stability and supramolecular approach of liquid/liquid extraction” F. Testard.
- Lecture to ICSM summer school (Marcoule, France) Août 07 : « Colloïdal approach in liquid/liquid extraction » F. Testard.
Book chapters:
- Chapter : « solubilization » (Zemb, Th., Testard, F.) in »Handbook of Applied colloïd and surface chemistry » editedb y Pr K. Holmberg (Wiley )., 159-188, (2002).
- Review chapter “ Third phase formation in liquid/liquid extraction: a colloidal approach” F. Testard, P. Bauduin, L. Berthon, Th. Zemb. in Ion Exchange and Solvent Extraction Edited by B. Moyer, CRC Press, chap 7, P381 (2009).
Responsabilities
| 2017-Today | Organising committee of GDR Or-Nano |
| 2014-Today | Chairperson of peer review committe 4 of synchrotron SOLEIL |
| 2014-Today | Member of the Concil of doctoral school « Interface, Université Paris-Saclay |
| 2012-2014 | Member of peer review committe 4 of synchrotron SOLEIL |
| 2011-2014 | Coordinator of MIGRANI ANR Project ANR-11_BS10_006. |
| 2009-2014 | Elected to ORGanisation of UsErs of SOLEIL (ORGUES)/ scientific committe of SOLEIL user’s meeting |
| 2005-2011 | Coordination in CEA of the students from the European Research Master: Colloïdal and supramolecular chemistry (UVSQ, University of Regensburg, University of Florence) |
Publications
2016:
– « Nanostructure changes upon polymerization of aqueous and organic phases in organized mixtures »
C. Noirjean; C. Vancaeyzeele, S. Bourcier, F. Testard, F. Vidal, D. Carriere; O. Fichet; Langmuir (2016), 32, 10104-10112.
– “Molten fatty acid based microemulsions”
C. Noirjean; F. Testard; C. Dejugnat; J. Jestin; D. Carriere; Phys. Chem. Chem. phys. (2016), 18, 15911-15918.
– » Uranium extraction by a bifunctionnal amido-phosphonic acid: coordination structure and aggregation »
O. Pecheur; D. Guillaumont; S. Dourdain, L. Berthon , R. Turgis, C. Fillaux, G. Arrachart, F. Testard, Solv. extr. ion Exch. (2016), 34, 260-273.
– « Synergism in a HDEHP/TOPO Liquid−Liquid Extraction System: An Intrinsic Ligands Property? »
O. Pecheur; S. Dourdain, D. Guillaumont, J. Rey, P. Guilbaud, L. Berthon, M.C. Charbonnel, S. Pellet-Rostaing, F. Testard J. Phys. Chem. B (2016), 120, 2814- 2823.
2015:
– “Gold Nanoparticle Internal Structure and Symmetry Probed by Unified Small-Angle X‑ray Scattering and X‑ray Diffraction Coupled with Molecular Dynamics Analysis”
B. Fleury, R. Cortes-Huerto, O. Taché, F. Testard, N. Menguy, and O. Spalla, Nanoletters (2015), 15, 6088−6094
– “Twinned Gold Nanoparticles under Growth: Bipyramids Shape Controlled by Environment”
Z. C. Z. Canbek, R. Cortes-Huerto, F. Testard, O. Spalla, S. Moldovan, O. Ersen, A. Wisnet, G.Wang, J. Goniakowski, C. Noguera, and N. Menguy, Cryst. Growth Des. (2015), 15, 3637−3644.
– « The role of solvent swelling in the self-assembly of squalene based nanomedicines »
D. Saha, F. Testard, I. Grillo, F. Zouhiri, D. Desmaele, A. Radulescu, S. Desert, A. Brulet, P. Couvreur and O. Spalla ; Soft Matter (2015) 1 4173-4179.
– “Recycling metals by controlled transfer of ionic species between complex fluids: en route to « ienaics »
Th. Zemb; C. Bauer; P. Bauduin; L. Belloni; C. Déjugnat; O. Diat; V.Dubois; J-F. Dufrêche; S. Dourdain; M. Duvail; C. Larpent; F. Testard; S. Pellet-Rostaing; Colloid Polym Sci (2015), 293 1-22.
2014:
– Quenched microemulsions: a new route to proton conductors”
C. Noirjean; F. Testard; J. Jestin; O. Tache; C. Dejugnat; D. Carriere; Soft Matter (2014), 10 5928-5935.
2013:
– “Micellization of dodecyltrimethylammonium bromide in water–dimethylsulfoxide mixtures: A multi-length scale approach in a model system”
V. Peyre; S. Bougerra; F. Testard, JCIS (2013), 389 164–174.
2012:
– « Understanding of the Size Control of Biocompatible Gold Nanoparticles in Millifluidic Channels« ,
Han J., Testard F., Malloggi F., Coulon P-E., Menguy N., Spalla O., Langmuir, (2012), 28 15966−15974.
– ”Surfactant (bi)layer on Gold Nanorods”,
Gomez-Grana, S.; Hubert, F.; Testard, F.; Guerrero-Martinez, A.; Grillo, I;. Liz-Marzan, L.M.; Spalla, O., Langmuir (2012), 28 1453-1459.
– “Growth and Overgrowth of Concentrated Gold Nanorods: Time Resolved SAXS and XANES”,
Hubert, F.; Testard, F.; Thill, A., Kong Q., Tache O., Spalla, O., Cryst. Growth Des. (2012), 12, 1548−1555
– “Synergism by Coassembly at the Origin of Ion Selectivity in Liquid-Liquid Extraction” ,
Dourdain, S; Hofmeister, I; Pecheur, O; Dufreche, JF; Turgis, R; Leydier, A; Jestin, J; Testard, F; Pellet-Rostaing, S; Zemb, T, Langmuir (2012), 28 11319-11328.
-“Micellization of dodecyltrimethylammonium bromide in water–dimethylsulfoxide mixtures: A multi-length scale approach in a model system”
V. Peyre; S. Bougerra; F. Testard, JCIS (2012), 389 (2013) 164–174.
“A Comprehensive Study of the Mechanism of Formation of Polyol-Made Hausmannite Nanoparticles: From Molecular Species to Solid Precipitation”
Rhadfi, T; Sicard, L; Testard, F; Tache, O; Atlamsani, A; Anxolabehere-Mallart, E Le Du, Y; Binet, L; Piquemal, JY , J Phys Chem C,(2012), 116 5516-5523
Before 2010:
– “Nanorods versus Nanospheres: A Bifurcation Mechanism Revealed by Principal Component TEM Analysis”,
Hubert F., Testard F., Rizza G., Spalla O., Langmuir (2010), 26, 6887-6891.
– “Influence of Monomer Feeding on a Fast Cold Nanoparticles Synthesis: Time-Resolved XANES and SAXS Experiments”,
Abecassis B., Testard F., Kong QY., Francois B, Spalla O. , Langmuir (2010), 26, 13847-13854.
– “Influence of the extracted solute on the aggregation of malonamide extractant in organic phases: Consequences for phase stability”
Berthon L, Testard F, Martinet L, Zemb T, Madic C., C. R. Chimie (2010) 13 1326–1334
– “Correspondence between Curvature, Packing Parameter, and Hydrophilic/Lipophilic Deviation Scales around the Phase-Inversion Temperature”,
Kunz, W., Testard, F., Zemb, Th., Langmuir, (2009), 25(1), 112-115.
– “Ternary phase diagrams of a thermoreversible chelating non-ionic surfactant”
Nave, S., Testard, F., Coulombeau, H., Baczko, K., Larpent, C., Zemb, Th., Phys. Chem. Chem. Phys., (2009), 11, 2700-2707.
– “Gold nanoparticle synthesis in worm-like catanionic micelles: microstructure conservation and temperature induced recovery”
B. Abécassis, F. Testard, Th. Zemb, Soft Matter, (2009), 5, 974-978.
– “Thermo-responsive Metal-Chelating Surfacants: Properties and use in cloud point extraction of uranyl nitrate”,
Prévost S., Coulombeau H., Baczko K., Zorz N., Desvaux H., Testard F., Zemb Th. And Larpent Ch., Tenside Surf. Det. (2009) 46, 100-104.
– “Self-assembling properties of malonamide extractants used in separation processes”
F. Testard F., Bauduin P., Martinet L., Abécassis B., Berthon L., Madic C., Zemb Th.. Radiochimica Acta, (2008) 96, 265-272.
– “Gold nanoparticle superlattice crystallization probed in situ”
Abecassis B., Testard F., and Spalla, O. Physical Review Letters (2008), 100 (11).
– “Solubilisation and interfacial curvature in microemulsions: I. Interfacial expansion and co-extraction of oil”
Tchakalova V., Testard F., Wong K., Parker A., Benczédi D. and Zemb Th., Colloids and Surfaces A: Physicochemical and Engineering Aspects (2008), 33, 31–39.
– “Solubilisation and interfacial curvature in microemulsions: II. Surfactant efficiency and PIT”
Tchakalova, V., Testard F., Wong K., Parker A., Benczédi D. and Zemb Th., Colloids and Surfaces A: Physicochemical and Engineering Aspects (2008), 331, 40–47.
– “Solubilization in alcanes by alcohols as reverse hydrotropes or « lipotropes”
Bauduin, P., Zemb, Th., Testard, F., J. Phys. Chem. B (2008) 112, 12354-12360.
– “Cetyltrimethylammonium Bromide Silver Bromide Complex as the Capping Agent of Gold Nanorods”
Hubert F., Testard F., Spalla O., Langmuir (2008) 24, 14, 9219-9222.
– “Probing in situ the Nucleation and Growth of Gold Nanoparticles by Small-Angle X-ray Scattering”
Abécassis B., Testard F., Spalla O., and Barboux Ph., Nanoletters (2007), vol 7, n°6, 1723-1727.
– “Relation between the hydrophile/hydrophobe ratio of malonamide extractants and the stability of the organic phase: investigation at high extractant concentrations »
Bauduin P., Testard F., Berthon L. and Zemb Th., Phys. Chem. Chem. Phys., (2007), 9, 3776 – 3785.
– » Nonionic Metal-Chelating Surfactants Mediated Solvent-Free Thermo-induced Separation of Uranyl”
Larpent C., Prévost S., Berthon L., Zemb Th., and Testard F., New Journal of Chemistry (2007), 31, 1424-1428.
– “Solvent penetration and sterical stabilization of reverse aggregates based on the Diamex process for extracting molecules: consequences for the third phase formation”
Berthon L., Martinet L., Testard F., Madic C., Zemb Th., Solv. Extr. Ion exchange, (2007), 25, 545-576.
– “Liquid-liquid extraction : an adsorption isotherm at divided interfaces ? »
Testard F., Berthon L., Zemb Th., C.R. Chimie (2007), 10, 1034-1041-
– « Electrostatic control of spontaneous curvature in catanionic reverse micelles”
Abécassis, B.; Testard, F.; Arleth, L.; Hansen, S.; Grillo, I.; Zemb, T., Langmuir (2007), 23, 9983-9989.
– “Futuristic back-end of the nuclear fuel cycle with the partitioning of minor actinides”
Madic C., Boullis B., Baron P., Testard F., Hudsonc M.J., Liljenzin J.-O., Christiansen B., Ferrando M., Facchini A., Geist A., Modolo G., Espartero A.G., De Mendoza J., Journal of Alloys and Compounds (2007) 444–445 23–27.
– “Phase Behavior, Topology, and Growth of Neutral Catanionic Reverse Micelles”
Abecassis, B.; Testard, F.; Arleth, L.; Hansen, S.; Grillo, I.; Zemb, T., Langmuir (2006); 22(19); 8017-8028.
– “Supramolecular organisation of tri-n butyl phosphate in organic diluent on approaching third phase transition”
Nave, S., Mandin, C., Martinet, L., Berthon, L., Testard, F., Madic, Ch., Zemb, Th., Phys. Chem. Chem. Phys., (2004), 6, 799 – 808
– “Effect of recognised and unrecognised salt on the self-assembly of new thermosensitive metal-chelating surfactants”
Coulombeau, H., Testard, F., Zemb, Th., Larpent, Ch., Langmuir (2004), 20, 4840-4850.
– “Aggregation Properties of TODGA in n-Dodecane”
Nave, S., Modolo, G., Madic, C., Testard, F., Solv. Extract., Ion Exch. (2004), 22, n°4, 527-551.
– “Analyse of the small angle scattering by a porous and granular medium”
Spalla O., Lyonnard S., Testard F., J. Appl. Cryst. (2003), 36, 338-347.
– “Effect of n-octanol on the structure at the supramolecular scale of concentrated malonamide extractant solutions”
Abecassis B., Testard F., Zemb Th., Berthon L., Madic Ch., Langmuir (2003), 19, 6638-6644.
– “How does ZrO2/surfactant mesophase nucleate? formation mechanism”,
Né F., Testard F., Zemb Th., Grillo I., Langmuir (2003), 19, 8503-8510.
– “Understanding solubilisation using principles of surfactant self-assembly as geometrical constraints”
F., Testard, Th. Zemb, C.R.A.S. Geoscience (2002), 334, 649-663.
– “Excess of solubilization of lindane in nonionic surfactant micelles and microemulsions”,
Testard F., Zemb Th., Langmuir (1998), 14, 3175-3181.
– “Excess of solubilization and curvature in nonionic microemulsions« ,
Testard, F., Zemb,Th., J. Colloid Int. Sci., (1999), 219, 11-19.
– “Solute effect on connectivity of w/o microemulsions”,
Testard, F., Zemb, Th., Langmuir, (2000) 16, 332-339.
– “Interpretation of phase diagrams: topological and thermodynamical constraints”
Testard F., Zemb Th., Colloids and Surfaces A, (2002), 205, 3-13.

