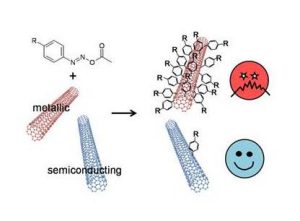
Carbon nanotubes are promising materials for many applications such as mechanics, electronics and optics. While nanotube reinforced composites are already entering the market, optical and electronic applications are slower to come because they require the use of semiconducting carbon nanotubes. Semiconducting nanotubes are synthesized within a mixture with highly conducting nanotubes, called metallic nanotubes. If a device is made with such a mixture, metallic nanotubes will cause short-circuits. Since the discovery of nanotubes, therefore, the separation of these two electronic types has been identified as a must-have condition before their use in industrial applications can be realized.
Several separation methods have been demonstrated in recent years, but they can only produce small amounts of purified material. Indeed, metallic and semiconducting nanotubes are chemically very similar, quite insoluble, and possess same length and diameter, both made solely of carbon atoms in a honeycomb lattice. Separation processes therefore must exploit tiny differences in their chemical affinity, which pushes the separation costs high; e.g., the density gradient ultra-centrifugation (1 mg of 99% separated nanotubes is sold at $900). Multiple purification steps are often necessary. Yet even an excellent quality semiconducting nanotubes with 99% purity still contains 1% of metallic nanotubes, a level still too high to eliminate short-circuits in electronic devices.
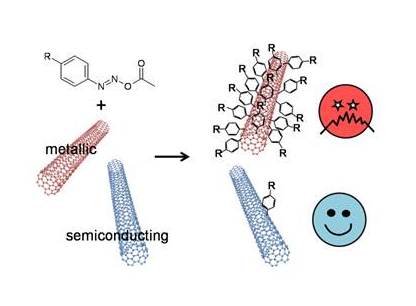
In search of a scalable chemical method, we chose diazonium that couples preferentially with metallic nanotubes in simple experimental conditions (room temperature, aqueous solution). This reaction has another advantage; namely, the diazonium-carbon nanotube coupling locally damages the nanotubes’ electronic structure and therefore its electrical conductivity. It would then be possible to selectively impair the metallic nanotube conductivity and remove the risk of short-circuits while the semiconducting nanotube conductivity remains intact without any separation steps. The cost of such semiconducting material preparation would be far smaller than separation of pure semiconducting nanotubes.
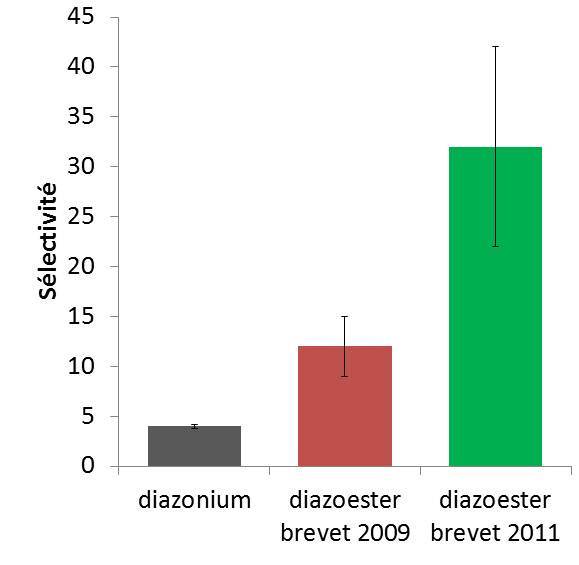
Although the diazonium-carbon nanotubes coupling has been known for over 10 years, it is scarcely used because of its selectivity that is too low to ensure a good separation. By studying this complex reaction mechanism in detail, we were able to demonstrate, for the first time, that it is a radical chain reaction (G. Schmidt et al, Chem. Eur. J. 2009). We also succeeded to identify the reaction step responsible for the metallic nanotube selectivity. Focusing on this crucial step, we considerably enhanced the coupling selectivity by replacing diazonium with another diazoic reagent; diazoester (G. Schmidt et al. Chem. Eur. J. 2011). This latter reagent is less redox active in solution, concentrates around the nanotubes in micellar media, and reacts with metallic nanotubes at least 10 times faster than it does with semiconducting nanotubes. Finally a recent innovation in the process increased the functionalization selectivity to > 20 on a series of carbon nanotube sources (CoMocat, HiPco, Laser and arc discharge).
In these conditions the diazo-coupling selectivity is sufficiently high to eliminate metallic nanotube conductivity while preserving the semiconducting nanotube quality. The reaction mixture can then be implemented directly into the fabrication of transistors without short-circuits. The process is now patented (P. Chenevier, Feb 6, 2009 and June 10, 2011) and used in solar cells and in printed electronics. In the first case, semiconducting carbon nanotubes are used as good electron acceptors that are mixed with organic electron donor dyes to produce active matrices of organic solar cells. In the second application, the low costing semiconducting material is an ideal candidate for inkjet electronic circuit printing.
References:
[1] G. Schmidt, S. Gallon, S. Esnouf, J.-P. Bourgoin, P. Chenevier Chemistry a European Journal 15, 2101 (2009)
[2] G. Schmidt, A. Filoramo, V. Derycke, J.-P. Bourgoin, P. Chenevier Chemistry a European Journal 17, 1415 (2011)
[3] “Procédé et kit de séparation de nanotubes de carbone métalliques et semiconducteurs”, P. Chenevier, Brevet 0950757, 6 fév. 2009.
[4] “Procédé de fonctionnalisation sélective des nanotubes de carbone monoparois”, P. Chenevier, Demande de brevet 1155137, 10 juin 2011.
Contact : Pascale Chenevier