One of the most important components of Li-ion batteries is the electrolyte, which consists of a salt dissolved in organic solvents. However, these solvents are toxic, environmentally harmful, and flammable. To address this issue, researchers are developing aqueous batteries. Yet, the low electrochemical stability of water (1.23 V in theory) limits the operating voltage of such batteries. To extend the stability window up to 3 V, highly concentrated aqueous electrolytes, known as water-in-salt (WISE) electrolytes, have been proposed. The interest of these systems lies notably in the modification of the local environment of water molecules, which limits the oxygen evolution reaction (OER) and the hydrogen evolution reaction (HER). The latter represents one of the major challenges for aqueous batteries. Indeed, hydrogen generation resulting from water reduction leads to capacity loss and reduced battery efficiency. Moreover, the lithium salts used in large quantities in these electrolytes are costly and often toxic. Switching to divalent ions such as magnesium could enable the development of more cost-effective and environmentally friendly electrolytes.
Researchers from the LEEL and LIONS teams at NIMBE investigated the stability of this type of electrolyte in full magnesium battery cells. In particular, they studied hydrogen production arising from the undesirable water reduction reaction in various magnesium-based aqueous electrolytes in order to assess their stability [1]. Contrary to what is often reported in the literature, the production of this unwanted gas mainly depends on the amount of salt (or water) present in the solution, rather than on the chemical nature of the electrolyte. This finding provides a key guideline for the design of more stable and higher-performance aqueous electrolytes.
One of the most critical components of Li-ion batteries is the electrolyte, which consists of a salt dissolved in non-aqueous organic solvents. The large amount of solvent required in these systems represents a major drawback, as the solvents commonly used are toxic, environmentally harmful, and highly flammable. A more sustainable alternative therefore consists in replacing these solvents with water. However, the intrinsically narrow thermodynamic stability window of water (1.23 V) severely limits its applicability. In this context, the researchers in this study developed highly concentrated aqueous electrolytes known as water-in-salt electrolytes (WISE), which enabled an extension of the electrochemical stability window up to 3 V [2].
The interest of this approach lies in the modification of the local environment of water molecules in solution and in the formation of a protective interphase at the electrode surface, which limits both the oxygen evolution reaction (OER) and the water reduction reaction, i.e. the hydrogen evolution reaction (HER). The latter constitutes one of the major challenges for aqueous batteries and has been the subject of extensive research and debate.
The sustainability of lithium resources is another major challenge, particularly in the case of WISE electrolytes, which require very large amounts of salt. Switching to divalent ions such as magnesium could therefore enable the development of more cost-effective and environmentally friendly concentrated electrolytes. However, the stability windows of magnesium-based aqueous electrolytes remain lower than those of lithium aqueous systems, which may originate from the limited solubility of magnesium salts in water. To overcome this limitation, several strategies have been proposed to reduce water reactivity, notably through the addition of molecular crowding agents such as polyethylene glycol (PEG). Another approach consists in introducing a co-salt, leading to so-called water-in-bisalt (WIBS) electrolytes, which reduce the availability of water molecules.
The common objective of these strategies is to strongly disrupt the organization of water molecules in solution in order to decrease their activity. So-called kosmotropic anions promote the hydrogen-bond network of water, whereas chaotropic anions disrupt this network. As a result, chaotropic anions are more effective at perturbing water structure and can theoretically further suppress the hydrogen evolution reaction. However, although these approaches have a significant impact on the structuring of water molecules in solution, their relevance for the long-term reactivity of aqueous batteries had not yet been clearly demonstrated.
In this study [1], the authors demonstrate that although the nature of the electrolyte strongly influences the hydrogen-bond network and ion-water interactions in solution, hydrogen production, and by extension electrolyte stability, is primarily governed by the water content of concentrated solutions.
To investigate reactivity in magnesium aqueous batteries, and in particular the role of electrolyte composition, the researchers compared electrolyte structuring using infrared spectroscopy and quantified hydrogen production by studying a range of magnesium electrolytes containing variable amounts of anions with distinct kosmotropic or chaotropic character. Infrared spectroscopy was used as the O-H stretching band of water provides valuable information on electrolyte structuring, especially with respect to the hydrogen-bond network and water–salt interactions. A shift of this band toward higher wavenumbers indicates weaker hydrogen bonds and increased disruption of the hydrogen-bond network by surrounding ions.
They showed that acetate-based solutions exhibit, regardless of concentration, an O-H stretching band shape comparable to that of pure water, reflecting the persistence of a highly structured hydrogen-bond network stabilized by this kosmotropic anion. In contrast, for chaotropic salts such as Mg(TFSI)₂ and Mg(ClO₄)₂, increasing molality leads to a shift of the O-H stretching band toward higher wavenumbers, indicating an overall weakening of the hydrogen-bond network. As molality increases, many water–water interactions are replaced by water-anion and water–cation interactions. Water molecules thus become increasingly involved in ion solvation, which should in principle limit their reactivity.
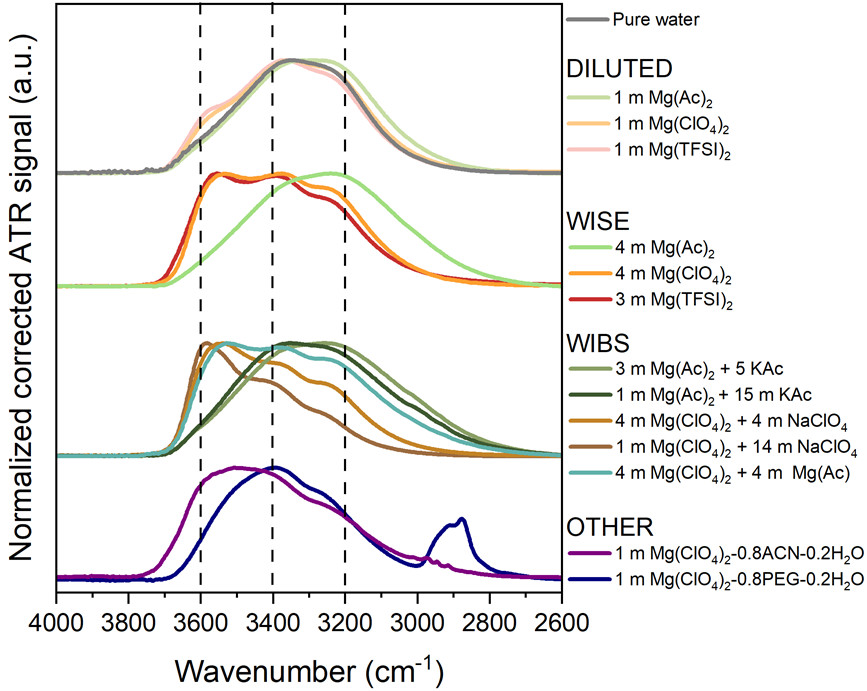
The O–H stretching bands obtained by infrared spectroscopy are shown for the different electrolytes investigated, including magnesium acetate, perchlorate, and imide salts at low and high concentrations. In addition, WIBS electrolytes with mixed cations were studied (magnesium acetate with potassium acetate, or magnesium perchlorate with sodium perchlorate). A mixed-anion WIBS electrolyte composed of magnesium acetate and magnesium perchlorate (Mg(CH₃COO)₂ and Mg(ClO₄)₂, denoted 4Mg4Mg) was also investigated. Finally, the addition of PEG and acetonitrile (ACN) to magnesium perchlorate solutions was evaluated.
To go beyond the influence of electrolyte nature on solution structuring, the authors also examined the effect of electrolyte composition on hydrogen (H₂) production resulting from water reduction. For this purpose, full cells were assembled using the different electrolytes in cells specifically designed for gas measurements (see figure below, left). The amount of gas produced was quantified by micro-gas chromatography after ten charge-discharge cycles.
Clearly, the nature of the anion has only a minor impact on H₂ production, in contrast to salt (or water) content. As illustrated in the figure below, H₂ production follows an identical dependence on salt weight fraction regardless of anion identity, indicating that the overall salt (or water) content, rather than the mere presence of free water molecules observable by infrared spectroscopy, primarily governs hydrogen evolution.
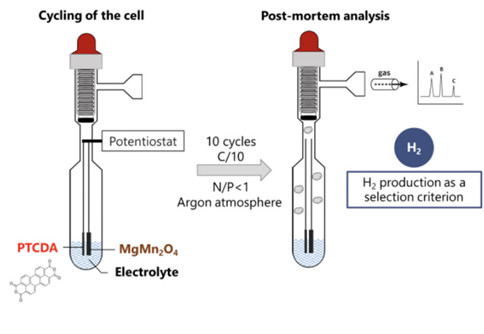
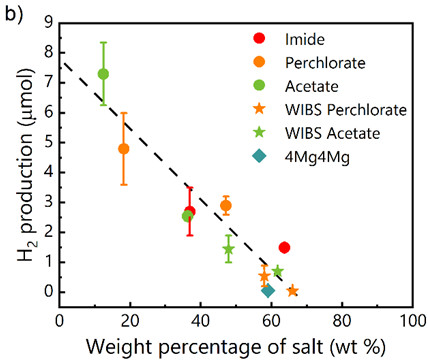
(left) Schematic of the modified ampoule designed to couple electrochemical measurements with gas quantification and of the methodology used in the study. (right) Hydrogen production after ten charge-discharge cycles as a function of salt percentage in different aqueous solutions.
In summary, hydrogen production decreases as salt concentration increases, but is primarily determined by the water content of concentrated solutions. Contrary to widely accepted assumptions in the literature, these results demonstrate that electrolyte structure has little influence on the reactivity of concentrated aqueous batteries. Comparative studies on other systems, whether monovalent or multivalent (Li, Na, Zn), would help assess the universality of this finding. Such an approach would be highly valuable for guiding the design of more efficient and sustainable concentrated aqueous batteries.
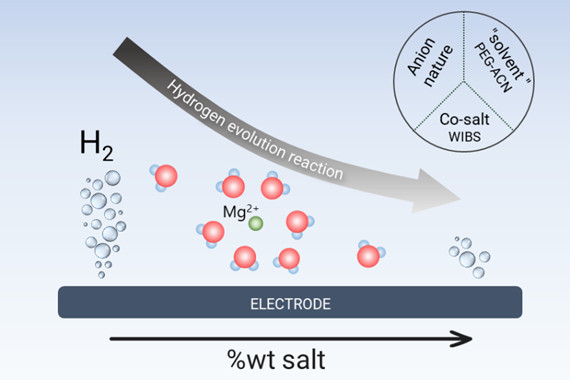
Illustration showing the direct correlation between salt (or water) content and undesirable hydrogen production in magnesium-based aqueous electrolytes, regardless of electrolyte nature.
References
[1] Is the Amount of Water the Most Important Parameter in Concentrated Aqueous Electrolytes? The Case of Aqueous Magnesium Cells, Malaurie Paillot, Sophie Le Caër, and Magali Gauthier, ACS Electrochemistry, 2025 1 (8), 1452-1461.
[2] “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries, Liumin Suo, Oleg Borodin, Tao Gao, Marco Olguin, Janet Ho, Xiulin Fan, Chao Luo, Chunsheng Wang, Kang Xu, Science 2015, 2015 350 (6263), 938.
Contacts CEA
- Magali Gauthier, NIMBE – Nanosciences et Innovation pour les Matériaux, la Biomédecine et l’Énergie, LEEL – Laboratoire d’Etude des Eléments Légers, CEA-IRAMIS.
- Sophie LE CAER, NIMBE – Nanosciences et Innovation pour les Matériaux, la Biomédecine et l’Énergie, LIONS – Laboratoire Interdisciplinaire sur l’Organisation Nanométrique et Supra Moléculaire, CEA-IRAMIS.