Marine Le Goas, Tom Roussel, Maria Kalbazova, David Carrière, Elodie Barruet, Valerie Geertsen, Giulia C. Fadda, Fabienne Testard, Geraldine Carrot and Jean-Philippe Renault
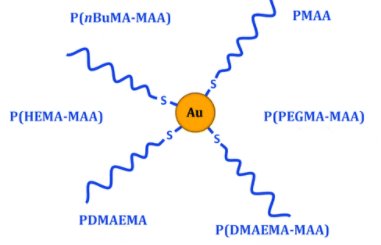
Nanomedicines are considered as promising therapeutics for cancer treatment. However, clinical translation is still scarce, partly because their biological behavior is not well understood. Extracting general guidelines from the great variety of nanoparticles and conditions studied is indeed difficult, and relevant techniques are lacking to obtain in situ information. Here, both issues are solved by combining versatile model nanoparticles with in situ tools based on small-angle scattering techniques (SAS). The strategy was to develop a library of nanoparticles and perform systematic study of their interactions with biological systems. Considering the promising properties of gold nanoparticles as cancer therapeutics, polymethacrylate-grafted gold nanoparticles were chosen as models. Modulation of polymer chemistry was shown to change the surface properties while keeping the same structure for all nanoparticles. This unity allowed reliable comparison to extract general principles, while the synthesis versatility enabled to fine-tune the nanoparticles surface properties, especially through copolymerization, and thus to optimize their biological behavior. Two specific aspects were particularly examined: colloidal stability and cell uptake. Positive charges and hydrophobicity were identified as key parameters influencing toxicity and internalization. In situ SAS gave valuable information about nanoparticles evolution in biologically relevant environments. Good colloidal stability was thereby shown in cell culture media, while intracellular transformation and quantity of nanoparticles were monitored, highlighting the potential of these techniques for nanomedicines studies.
https://doi.org/10.1039/d0tb01167c