Christophe FAJOLLES
Recently, it has been theoretically proposed that polyrotaxanes, the inclusion complexes formed between a polymer and a ring-like molecule, could lead to a new class of grafted polymer layers whereby the attachment point between the chain and the substrate would allow the chains to slide freely, while being prevented from escaping by a capping group at each chain-end chosen to allow biorecognition. This form of chain attachment to a surface leads to a new class of polymer interfacial structure, that we name Sliding Anchored Polymers, or SAPs, with many new interesting potential features from the point of view of both equilibrium and dynamic layer properties.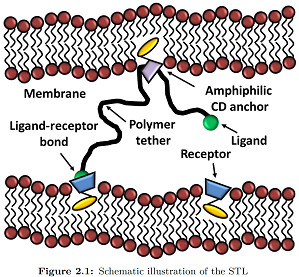
In our demonstration kit, the surface is a self-assembled lipid bilayer; the ring is a alpha cyclodextrin functionalized with a cholesterol moiety; the ligand is an adamantine, a molecule that makes an inclusion complex with anchored beta cyclodextrins.
First amphiphilic cholesteryl cyclodextrins have been synthesized and investigated from the macroscopic to the molecular scale. Langmuir, neutron reflectivity and other surface techniques have been used to show that the functionalized cyclodextrin rings form stable monolayers, and that the orientation of the sliding cavity depends on the surface density[1][2]. Once validated, these methods have been applied to the new synthesized SAPs. Especially, using Infra Red Reflection Absorption Spectroscopy (IRRAS) we demonstrate that these new sliding polymer complexes anchor well in model membranes self-assembled from DPPC. Then, using neutron reflectivity, we show that for sufficiently high polymer densities the SAPs inserted into lipid monolayers and lipid bilayers form polymer brushes, consistent with theoretical predictions for polymers with a sliding anchor.[3] Finally, as a proof of concept, the surface adhesion properties induced by these new nano-objects were measured by surface force measurements, showing forces due to the sliding tethered ligand-receptor interactions. These key steps have still to be fully described, but have surely contributed to the Prize attributed to the associated PhD thesis.
These SAPs came as a natural yet challenging continuation of our long term interest in amphiphilic CDs and their interactions with membranes as well as CDs’ inclusion compounds. Dicholesteryl-CDs and Leucinyl-CDs are recent exemples[4][5]. The project was supported by ANR Pnano and Région Alsace.
Moreover, the acquired experience allowed us to start the ANR blanc SIMI 9 project FibRotaxanes entitled “Multi-Active and Nanostructured Polyrotaxane-based Nano-Fibers: A new strategy for Tissue Engineering”.
Central to this project, the first SAPs have been synthesised, and the induced forces have been measured. Indeed, a synthetic pathway for the assembly of the SAP’s had to be developed. In particular polymers complexed with a very low number of modified rings and end-capped with a ligand were obtained by a new combination of chemical and self-assembly techniques.
[1] M. Bauer, C. Fajolles, T. Charitat, H. Wacklin, J. Daillant J. Phys. Chem. B 115, 15263–15270, 2011.
[2] M. Bauer, T. Charitat, C. Fajolles, G. Fragneto, J. Daillant Soft Matter 8, 942–953, 2012.
[3] M. Bauer, M. Bernhardt, T. Charitat, P. Kekicheff, C. Fajolles, G. Fragneto, C. M. Marques, J. Daillant Soft Matter 9, 1700–1710, 2013.
[4] A. Klaus, C. Fajolles, M. Bauer, M. Collot, J.-M. Mallet, J. Daillant Langmuir 27, 7580–7586, 2011.
[5] M. Roux, E. Sternin, V. Bonnet, C. Fajolles, F. Djedaini-Pilard Langmuir 29, 3677–3687, 2013.




