Innovative energy storage based on magnesium and aqueous electrolytes
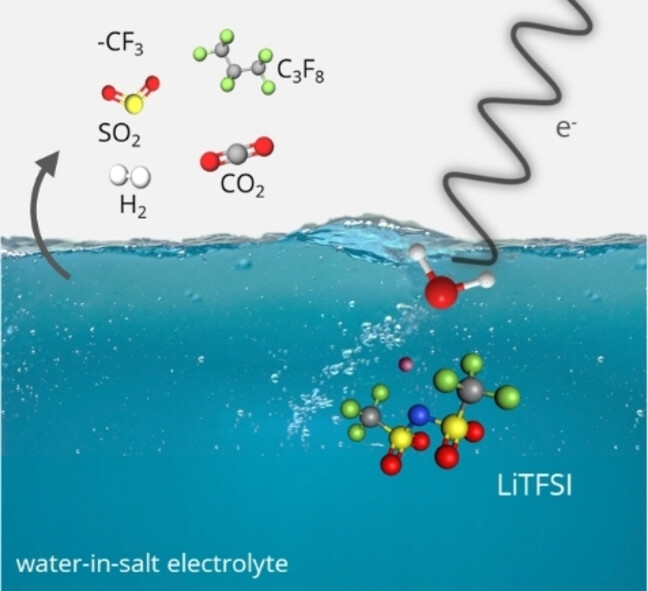
Aqueous solutions play a key role in biology, chemistry and, more particularly, in energy-related fields such as photo and electro-catalysis and batteries. Whereas aqueous batteries are supposed to be constrained by the thermodynamic electrochemical stability window of water (1.23 V vs. NHE under standard conditions in the absence of overvoltage), the concept of highly salt-concentrated aqueous electrolytes (WISEs) leads to an impressive increase in the potential window of aqueous lithium batteries. This is mainly due to the absence of free water molecules and the crucial role of the salt anion-based electrode/electrolyte interface in the layer described by Helmholtz. While WISEs open the way to competitive and sustainable systems, these solutions currently use costly and toxic salts, thus hampering their use on an industrial scale. This technological bottleneck is also associated with the lack of fundamental knowledge underlying the WISE concept, which has yet to be rationalized for a wider range of salts, ions and concentrations. Multivalent cations and associated salts are an interesting alternative, particularly those based on magnesium, which is safer, cheaper and more abundant than lithium.
However, the only aqueous Mg battery based on WISEs currently reported in the literature has been tested over an optimized potential window of 2 V, thus not offering a very wide increase in its range of use and without offering a clear understanding of the mechanisms involved. Consequently, this project targets the potential window of aqueous rechargeable Mg batteries and aims to develop innovative and more sustainable electrolyte solutions for Mg batteries. Above all, understanding the role of the electrolyte and controlling phenomena at interfaces is essential, and requires fundamental knowledge of WISEs reactivity.
The main objective of MAGWAT is to determine whether aqueous Mg batteries based on concentrated salts are a viable future technology. Specifically, the aims of the project are: 1) to gain a fundamental understanding of the reactivity of concentrated aqueous solutions based on Mg salts compared with those based on Li, 2) to understand the phenomena occurring at the heart of the solutions and at the interfaces, and 3) to propose and develop innovative electrolytes to overcome the low solubility of Mg salts in water. This will be achieved firstly through a multi-scale approach to understanding, involving pioneering experiments such as radiolysis and synchrotron X-ray spectroscopy coupled with theoretical calculations, from which the critical characteristic quantities of electrolytes will be extracted. This fundamental knowledge will then be used to design innovative electrolyte solutions. With this approach, we plan to increase the potential window of aqueous rechargeable Mg batteries and thus target the development of suitable positive and negative electrodes.
Thus, by developing rechargeable batteries based on Mg and water, this project will aim to develop more ecologically sustainable and safe batteries, using more abundant and less toxic materials than today’s Li-ion batteries. In addition, advances in fundamental knowledge and innovative strategies will have an impact on other battery chemistries and energy storage-related fields such as supercapacitors and catalysis, paving the way for greener, safer storage.
Project coordination
Magali GAUTHIER (NIMBE\LEEL)
Collaborator
Sophie le Caer (NIMBE\LIONS)
PCM2E Laboratory of Physico-Chemistry of Materials and Electrolytes for Energy





