The safety of radioactive waste storage notably relies on controlling hydrogen (H₂) generation arising from the radiolysis of cementitious materials. Until now, most studies have focused on the pore water of cement matrices, largely overlooking the role of the solid phases themselves. Recent work carried out at LIONS, NIMBE (UMR CEA, CNRS), in collaboration with researchers from DES of CEA (LECBA), has elucidated the elementary mechanisms of radiolysis in model minerals representative of cement paste: portlandite and tobermorites. By combining irradiation experiments, spectroscopy, and simulations, this research provides key insights for quantifying and modeling hydrogen-related risks in cement-based structures exposed to ionizing radiation.
Cementitious matrices used for the conditioning of intermediate-level long-lived radioactive waste (ILW-LL) are exposed to ionizing radiation, which may lead to the generation of radiolytic dihydrogen (H₂). The accumulation of dihydrogen poses a safety concern when its concentration in air exceeds 4%, making its production a parameter that must be reliably modeled. Radiolytic H₂ generation is generally estimated based on the residual interstitial (pore) water present in cementitious materials, thereby neglecting any potential contribution from the solid phases. However, radiolysis in these materials is a complex process that must be fully accounted for. In this context, the doctoral work of Thibaut Hérin demonstrates that hydrated solid phases of cement can also contribute—albeit to a lesser extent—to H₂ production through radiolytic mechanisms specific to their composition and structure.
To better quantify this contribution, three minerals representative of cement hydrates were specifically investigated under irradiation: portlandite, 9 Å tobermorite, and 11 Å tobermorite. This selection made it possible to separately examine the radiolytic behavior of structural water (OH groups) and crystallization water (H₂O molecules). Prior to irradiation, the samples were carefully desorbed to eliminate any parasitic contribution from adsorbed water under ionizing radiation. Irradiations using accelerated electrons (ALIENOR accelerator) and γ-rays revealed mineral-dependent H₂ production.
Results obtained for portlandite (Ca(OH)₂, Figure 1) highlighted a dual mechanism:
- an immediate production of dihydrogen at the material surface, arising from the recombination of radical species;
- a delayed production resulting from the release of dihydrogen trapped within the crystal lattice, which is gradually liberated over several days or weeks following irradiation (Figure 1).
In addition, electron paramagnetic resonance (EPR) measurements revealed the presence of transient radical species (CaO•, H•) and enabled identification of the reaction pathways responsible for these phenomena. These observations were supported by simulations performed at LECBA, which showed that dihydrogen diffusion in portlandite is anisotropic and subdiffusive, accounting for the slow post-irradiation release of the gas.
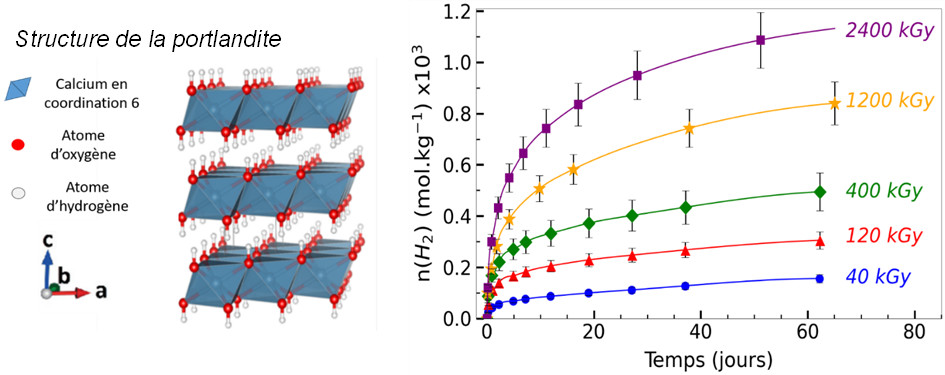
Figure 1. Left: crystal structure of portlandite; right: temporal evolution of delayed H₂ release from portlandite as a function of time and irradiation dose delivered to the material, expressed in Gray (Gy, with 1 Gy = 1 J·kg⁻¹).
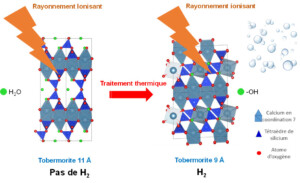
Complementary studies on tobermorites—structural models of calcium silicate hydrates (C–S–H, the main mineral phase formed during cement hydration)—showed that only surface Si–OH groups contribute to dihydrogen production, whereas crystallization water within the bulk solid does not generate gas in these minerals. Indeed, 9 Å tobermorite (Ca₅Si₆O₁₆(OH)₂), although the least hydrated, exhibited the highest H₂ production, through a surface-localized mechanism. In contrast, experiments on 11 Å tobermorite (Ca₅Si₆O₁₇.₅·H₂O) demonstrated that radiolysis of crystallization water does not lead to dihydrogen formation; instead, the generated radicals preferentially form new Si–OH bonds. This result is particularly noteworthy, as 11 Å tobermorite does not produce dihydrogen under irradiation. Upon heating, it is transformed into 9 Å tobermorite, which does produce H₂ (Figure 2).
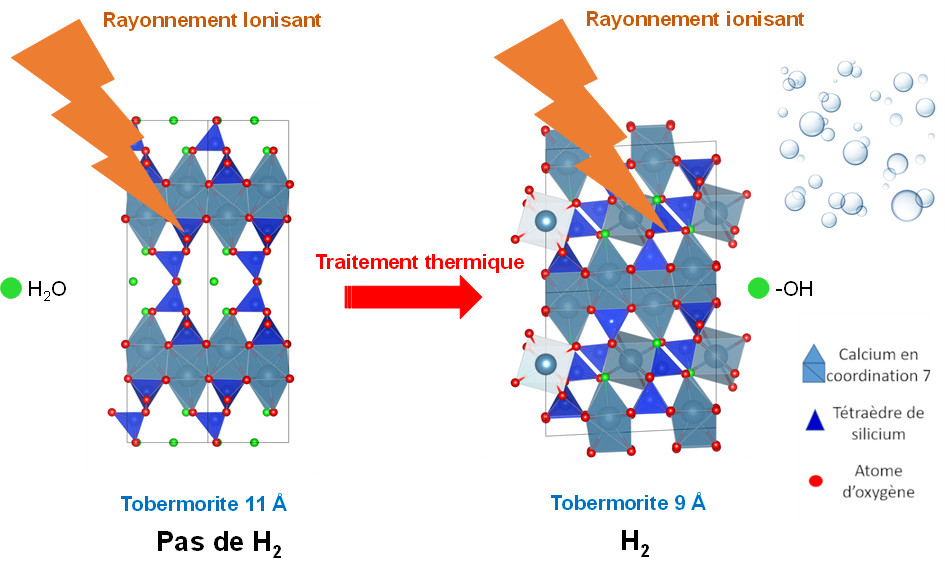
Figure 2. 9 Å tobermorite, which contains O–H bonds, produces H₂ under ionizing radiation. It is obtained by thermal treatment of 11 Å tobermorite, which contains crystallization water molecules and does not generate dihydrogen under irradiation.
By combining irradiation experiments, spectroscopic characterizations, and molecular modeling, this body of work provides a comprehensive view of radiolysis mechanisms in selected cementitious solids of interest. It demonstrates that the contribution of the solid phase to H₂ production cannot be neglected, while also clarifying its origin (surface vs. bulk) and kinetics (immediate vs. delayed). These results indicate that radiolytic dihydrogen (H₂) production in cementitious solids is predominantly governed by surface processes. In real cement matrices, where the pore network is largely water-saturated, this contribution is expected to remain limited compared to that arising from pore water radiolysis. Funded by EDF, this work provides essential inputs to improve the operational models used by the industrial partner to assess the H₂ source term associated with cemented waste. It thus refines the understanding of hydrogen-related risks in cement-based radioactive waste conditioning and is being further extended within the framework of the PhD project of Mathilde Linger.
References
[1] T. Herin, T. Charpentier, P. Bouniol, S. Le Caër, Behavior of Portlandite upon Exposure to Ionizing Radiation: Evidence of Delayed H₂ Production, J. Phys. Chem. C, (2023).
[2] T. Honorio, M. Trifa, T. Herin, S. Le Caër, H₂ Anisotropic Subdiffusion and Induced Expansion in Portlandite, Gibbsite, and Boehmite, J. Phys. Chem. C, (2024).
[3] T. Herin, T. Charpentier, P. Bouniol, S. Le Caër, H₂ Production Mechanisms in Irradiated Portlandite: Surface and Volume Contributions, J. Phys. Chem. C, (2024).
[4] T. Herin, A. Alessi, T. Charpentier, S. Poyet, P. Bouniol, S. Le Caër, Reactivity of Constitution vs. Crystallization Water under Irradiation: Insights from Tobermorites, Int. J. Hydrogen Energy, (2025).
[5] T. Herin, Mécanismes de la radiolyse dans les hydrates cimentaires et conséquence sur la formation de dihydrogène dans les matériaux irradiés, thèse de l’Université Paris-Saclay (2024).
Contact CEA
Sophie LE CAER, NIMBE – Nanosciences et Innovation pour les Matériaux, la Biomédecine et l’Énergie, LIONS – Laboratoire Interdisciplinaire sur l’Organisation Nanométrique et Supra Moléculaire, CEA-IRAMIS.