Hydrogen has a high mass energy density of 120 MJ/kg, almost three times that of hydrocarbons. This characteristic makes hydrogen an excellent energy storage medium, particularly in the context of an energy transition based on renewable low-carbon energies (solar, wind). However, hydrogen has a low volumetric energy density, and needs to be concentrated in volume, pending its use. Conventional storage methods in gaseous form (compression) or liquid form (low-temperature liquefaction) have their limits in terms of efficiency, and require specific safety precautions.
Chemical storage is another way to reversibly store hydrogen. Boron hydrides, with their high energy density and thermal stability, are attractive for these energy storage applications, but their use in cycles requires the ability to regenerate them.
The NIMBE/LCMCE team is proposing a new synthesis route applicable to the regeneration of boron hydrides, by hydrogenolysis of chlorinated precursors, intermediate products in the cycle of hydrogen storage in boron-based materials. This method is based on “mild” conditions: 25°C and 10 bar of hydrogen, as compared with the industrial processes on which boron hydride synthesis is based today.
Chemical storage, which binds hydrogen to other molecules, facilitates its transport and use, and boron hydrides are an innovative solution for reversible hydrogen storage. Their high energy density and thermal stability make them attractive for mobile and stationary applications, such as fuel cell cars and renewable energy storage systems. Although the release of hydrogen from these materials is well studied, no process is yet operational. One of the technological hurdles to the deployment of this technology, on which research efforts are continuing, is the regeneration of the boron hydrides, formed from the by-products (boron oxides, boron nitride BN) resulting from the release of hydrogen. One of the necessary steps to achieve a sustainable and efficient use of these materials.
Boron hydrides, notably ammonia-borane (NH3-BH3) and borohydrides (BH4–), in particular alkali metal hydrides (LiBH4, NaBH4), are stable materials capable of storing large quantities of hydrogen by mass (19.5% wtH2 for NH3-BH3, 19 wtH2% for LiBH4). Currently widely used for their reducing properties in many industrial processes and in synthetic chemistry, their use for hydrogen storage has been envisaged with the development of fuel cells, but is hampered by the challenge of their synthesis: on an industrial scale, the preparation of boron hydrides relies mainly on high-temperature processes of the Brown-Schlesinger or Bayer type, which require the use of alkali metals (sodium metal) that weigh heavily on the energy balance. The very low energy efficiency of these processes makes it impossible to envisage reversible hydrogen storage using this process. This also implies the regeneration of by-products from hydrogen extraction, such as borazines (B3N3H6) or boron oxides.
As part of Guilhem Zwart’s thesis at the Laboratoire de Chimie Moléculaire et de Catalyse pour l’Énergie (NIMBE/LCMCE), the team worked on one of the stages in this regeneration, with the first synthesis of boron hydrides by hydrogenolysis of chlorinated precursors under mild conditions: 25°C and 10 bar of hydrogen. This method avoids the use of strong reducing agents and relies on the activation of hydrogen by the boron compound using triethylamine (Et3N : N(CH2CH3)3), an abundant base. This method was initially developed and optimized on dialkylchloroborane substrates (R2BCl, where R is an alkyl) before being adapted for the preparation of higher value compounds such as catecholborane (C6H4O2BH) and pinacolborane ((CH3)4C2O2BH) used in organic synthesis for the reduction of C=O bonds. [1]
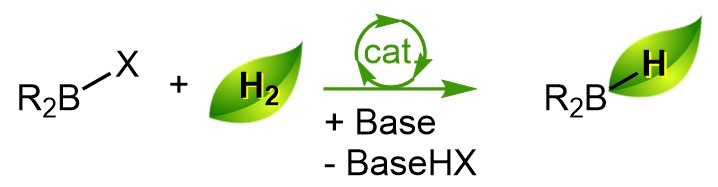
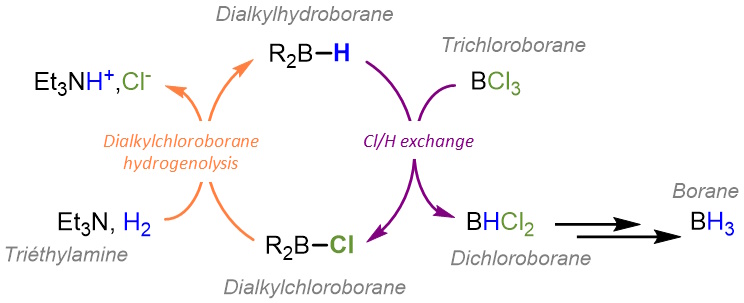
Trichloroborane (BCl3) in the presence of triethylamine does not activate hydrogen under the conditions initially described, but exchanges of halide/hydride groups on boron derivatives are known to occur. Based on this result, the team developed a catalytic system based on dialkylchloroboranes (R2B-Cl). In a first step, dialkylchloroborane is converted to dialkylhydroborane (R2B-H) by hydrogenolysis, i.e. by breaking the B-Cl bond through the action of hydrogen, in the presence of triethylamine. A chloride/hydrogen exchange between BCl3 and dialkylhydroboranes (R2B-H) leads to the intermediates dichloroborane (HBCl2) and chloroborane (H2BCl), as well as regenerating dialkylchloroborane from dialkylhydroborane to initiate a new catalytic cycle. However, this method requires working under harsher pressure and temperature conditions, up to 140°C and 50 bar of hydrogen, but leads to the selective obtention of dichloroborane (HBCl2) or chloroborane (H2BCl), two intermediates in the synthesis of borane (BH3), the latter being observed only in small proportions (5%).
These results represent a breakthrough for the production of boron hydrides, an element which can be used to fix hydrogen in an easily reversible way. They have been the subject of a patent application, as well as an article in the journal Angewandte Chemie. They have also paved the way for new collaborative projects, with Lilian Hoareau’s thesis funded by PEPR H2 to pursue research into the synthesis of new renewable hydrides for hydrogen storage.
References:
[1] “Hydrogenolysis of haloboranes: from synthesis of hydroboranes to formal hydroboration reactions”
G. Zwart, A. Mifleur, G. Durin, E. Nicolas, T. Cantat, Angew. Chem. Int. Ed. 2024 e202411468
[2] Guilhem Zwart thesis (2024) : “Hydrogenolysis of (pseudo-)haloboranes and chlorophosphines“.
Contact CEA-IRAMIS: Alexis Mifleur, Laboratoire LCMCE du NIMBE (UMR CEA-CNRS), Université Paris-Saclay.
Nomenclature of chemicals encountered in the synthesis :
- Ethyl group : –CH2CH3
- Trié^ethylamine (NEt3 : N(CH2CH3)3 ),
- Dialkylchloroboranes (R2BCl)
- Chloroborane (H2BCl)
- Trichloroborane (BCl3)
- Dichloroborane (HBCl2)
- Borane (BH3)
- Ammonia-borane (NH3.BH3)
- Borohydrure (BH4−)
- Borazines (B₃N₃H₆)
- Catecholborane (C6H4O2BH)
- Pinacolborane ((CH3)4C2O2BH)

