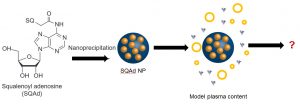
Caracterization of the future of organic nanodrugs in biological media
The Nanoprotection research project (http://nanoprotection.cnrs.fr/) gathers six French laboratories. Four scientist from the LIONS are involved: Frédéric Gobeaux, Jean-Philippe Renault, Fabienne Testard and Patrick Guenoun. This project aims at developping anew therapeutical approach through squalenoyl nanoparticles to treat neurodegenrative diseases : Charcot Marie-Tooth 1A and lesions caused by trauma in the brachial plexus.
Nucleoside squalenization has been developed by Patrick Couvreur’s team (Institut Galien, Faculté de Pharmacie de Chatenay-Malabry) in 2006. This approach allows synthesizing aqueous suspensions of nanoparticles made of active principle through the simple and energy-cheap nanoprecipitation process.1,2 These nanoparticles enable to reach a high encapsulation rate. They furthermore protect the active principle from a rapid degradation and allow targeting spatially and temporally its delivery.
The aim of our team in the Nanoprotection project is to study the structure and the future of Squalene-Adenosine (Sq-Ad) nanoparticles in biological media. Today, little is known on the structure of organic nanodrugs (liposomes, vesicles, nanoparticles…) in serum or cytoplasm. The main obstacle in characterizing the objects in situ comes from the similarity in terms of atoms and functions between them and the biological medium. An even higher degree of complexity is reached when the particles (like Sq-Ad NPs) are labile objects.
It is thus necessary to characterize Sq-Ad nanoparticles before and after their injection to fully control their preparation and understand their future in the organism. Our contribution is to develop strategies to monitor in vitro the structure and size distribution of nanoparticles in a model biological medium. We use our expertise in dispersed and complex media to specifically study these biologically active nanoparticles in biological media.3–5
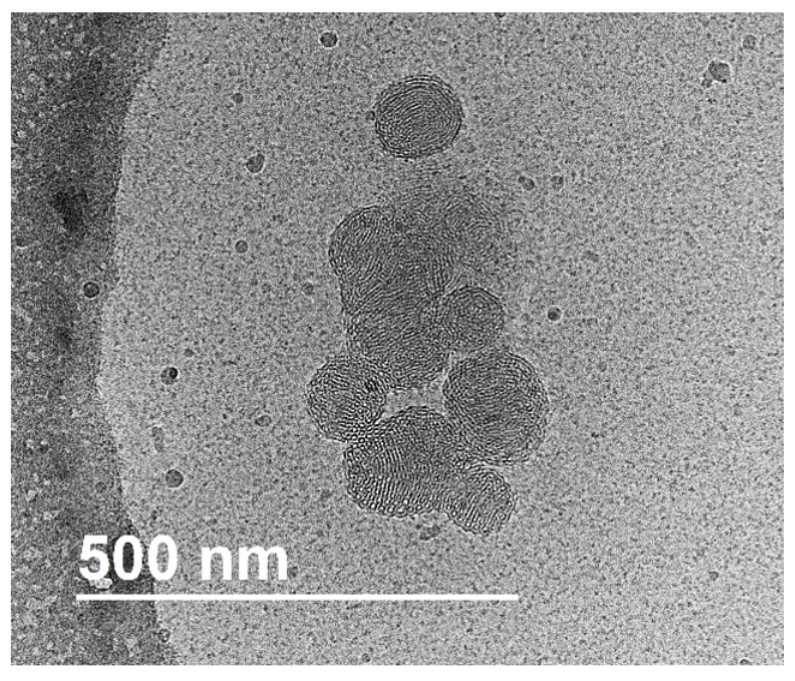
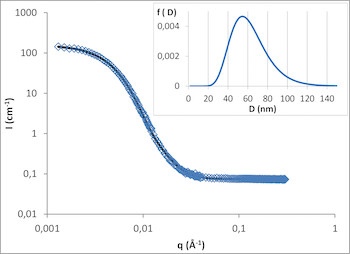
Among the different available characterization techniques, Small Angle Neutron Scattering (SANS) enables to monitor density fluctuation at the nanometer scale. Recently, we have demonstrated the efficiency of this approach to follow the formation steps of other squalenoyl nanoparticles (Squalene-Gemcitabine and Squalene-Deoxytidine).6 We have thus explained the size-distribution control through solvent activity and the existence of an internal structure right from the first steps of formation.
We are building on these first results to study the structure of Sq-Ad nanoparticles and develop specific methodologies to monitor their evolution in model biological media. The first step consistd in monitoring the nanoparticles in a deuterated medium and then to deuterate a part of the Sq-Ad molecule to study it in a hydrogenated serum. In parallel, we study the specific interaction between Sq-Ad nanoparticles and chosen serum constituents (albumins, lipoproteins, etc.) with different spectroscopic and calorimetric techniques
References
(1) Squalenoyl Nanomedicines as Potential Therapeutics.
Couvreur, P.; Stella, B.; Reddy, L. H.; Hillaireau, H.; Dubernet, C.; Desmaële, D.; Lepêtre-Mouelhi, S.; Rocco, F.; Dereuddre-Bosquet, N.; Clayette, P.; et al. Nano Lett. 2006, 6 (11), 2544–2548.
(2) Discovery of New Hexagonal Supramolecular Nanostructures Formed by Squalenoylation of an Anticancer Nucleoside Analogue.
Couvreur, P.; Reddy, L. H.; Mangenot, S.; Poupaert, J. H.; Desmaële, D.; Lepêtre-Mouelhi, S.; Pili, B.; Bourgaux, C.; Amenitsch, H.; Ollivon, M. Small 2008, 4 (2), 247–253.
(3) Structural Determinants for Protein Adsorption/Non-Adsorption to Silica Surface.
Mathé, C.; Devineau, S.; Aude, J.-C.; Lagniel, G.; Chédin, S.; Legros, V.; Mathon, M.-H.; Renault, J.-P.; Pin, S.; Boulard, Y.; et al. PLoS ONE 2013, 8 (11), e81346.
(4) Interferences of Silica Nanoparticles in Green Fluorescent Protein Folding Processes.
Klein, G.; Devineau, S.; Aude, J. C.; Boulard, Y.; Pasquier, H.; Labarre, J.; Pin, S.; Renault, J. P. Langmuir 2016, 32 (1), 195–202.
(5) Structure and Function of Adsorbed Hemoglobin on Silica Nanoparticles: Relationship between the Adsorption Process and the Oxygen Binding Properties. Devineau, S.; Zargarian, L.; Renault, J. P.; Pin, S. Langmuir 2017, 33 (13), 3241–3252.
(6) The Role of Solvent Swelling in the Self-Assembly of Squalene Based Nanomedicines.
Saha, D.; Testard, F.; Grillo, I.; Zouhiri, F.; Desmaele, D.; Radulescu, A.; Desert, S.; Brulet, A.; Couvreur, P.; Spalla, O. Soft Matter 2015, 11 (21), 4173–4179.
(7) Measuring Protein Structure and Stability of Protein–Nanoparticle Systems with Synchrotron Radiation Circular Dichroism.
Laera, S.; Ceccone, G.; Rossi, F.; Gilliland, D.; Hussain, R.; Siligardi, G.; Calzolai, L. Nano Lett. 2011, 11 (10), 4480–4484.
(8) Sensing of Hydrophobic Cavity of Serum Albumin by an Adenosine Analogue: Fluorescence Correlation and Ensemble Spectroscopic Studies.
Nag, M.; Bera, K.; Chakraborty, S.; Basak, S. J. Photochem. Photobiol. B 2013, 127, 202–211.
(9) Interaction of Bovine Serum Albumin with Self-Assembled Nanoparticles of 6-O-Cholesterol Modified Chitosan.
Li, X.; Chen, M.; Yang, W.; Zhou, Z.; Liu, L.; Zhang, Q. Colloids Surf. B Biointerfaces 2012, 92, 136–141.
(10) Interaction of Glutathione with Bovine Serum Albumin: Spectroscopy and Molecular Docking.
Jahanban-Esfahlan, A.; Panahi-Azar, V. Food Chem. 2016, 202, 426–431.
© Ce projet est soutenu par une subvention publique supervisée par l'Agence Nationale de la Recherche (ANR) dans le cadre du programme 'Investissements d'Avenir' (Labex NanoSaclay, référence: ANR-10-LABX-0035), coordonnée par Liliane Massade.