P. Maillet*, C. Levard**, E. Larquet§, C. Mariet,* O. Spalla*, N. Menguy§, A. Masion**, E. Doelsch‡, J. Rose** et Antoine Thill*
Imogolites (OH)3Al2O3Si(OH) are natural minerals discovered in 1962 in Japanese volcanic soils. Their structure is similar to that of a carbon nanotube. They are made of a curved Gibbsite sheet Al(OH)3 forming a nanotube of 2 nm in diameter. The tetrahedral silicon adsorbed inside the nanotube controls its curvature. The diificulties to synthesized large quantities of this mineral have indered the development of industrial applications. Recently a new synthetic process has allowed to circumvent this difficulty. Research are now performed to better understand and control this new synthesis.

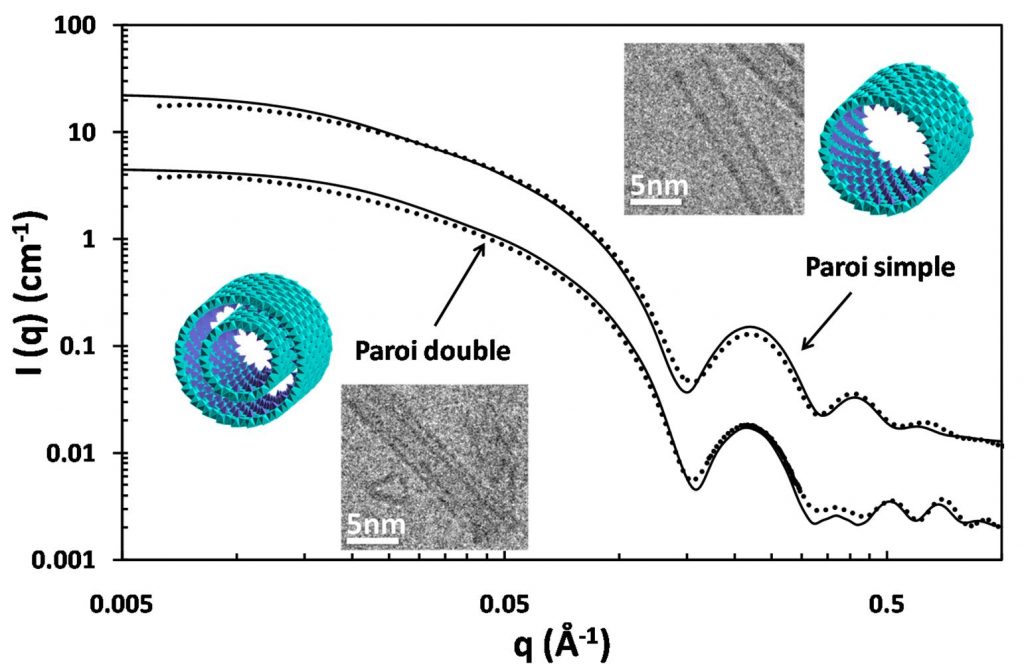
The increase of the synthesis yield was made possible by replacing silicon with germanium. Indeed, a CNRS (CEREGE, Aix-en-Provence) team was able, to synthesize large quantities of Al-Ge Imogolite analogues [1]. The structure of these analogues has been analyzed in detail by Small Angle X-ray Scattering (SAXS) at the ESRF BM02 beamline and by cryo-TEM by a team of IRAMIS-SIS2M. It is shown that these analogues are Imogolite nanotubes, but they exist in two forms. Depending on the synthesis conditions, it is possible to produce single-walled tubes of 3.5 nm in diameter, but also double-walled tubes of 4 nm diameter. Such double-walled Imogolite-like nanotubes are reported for the first time. The high reactant concentration used for this synthesis also unraveled the controversial nature of the so called proto-Imogolite which was poorly known so far.
This new synthesis is the first that enables the production of large quantities of monodisperse nanotubes with a diameter less than 5 nm, perfectly calibrated, hydrophilic and prone to functionalization. The current research aims firstly at understanding the mechanisms and kinetics of formation of these new nanotubes and also at controling their organization. In parallel, the toxicity study is carried on in our laboratory.
This work is the subject of a publication in the “Journal of the Chemical Society (JACS)” [2]
Reference:
[1] C. Levard, J. Rose, A; Masion, E. Doelsch, D. Borschneck, L. Olivi, C. Dominici, O. Grauby, J.C. Woicik and J.-Y. Bottero, J. Am. Chem. Soc. 2008, 130 (18) 5862.
|
[2] Evidence of Double-Walled Al-Ge Imogolite-Like Nanotubes. A Cryo-TEM and SAXS Investigation, P. Maillet, C. Levard, E. Larquet, C. Mariet, O. Spalla, N. Menguy, A. Masion, E. Doelsch, J. Rose and A. Thill, J. Am. Chem. Soc, 132 (2010) 1208. |
-
*CEA, IRAMIS- SIS2M/Laboratoire Interdisciplinaire sur l’Organisation Nanométrique et Supramoléculaire (LIONS)
91191 Gif-sur-Yvette, France. -
**CEREGE, UMR 6635 CNRS/Université Aix Marseille,
Europôle Méditerranéen de l’Arbois, 13545 Aix en Provence, France. -
§Institut de Minéréalogie et Physique des Milieux Condensés UMR 7590,
CNRS, Université Pierre et Marie Curie, Université Paris Diderot,
Institut de Physique du Globe de Paris, 140 rue de Lourmel, 75015 Paris, France, - ‡CIRAD, UPR Recyclage et risque, F-34398 Montpellier, France




