Chemists of DSV (CEA-Life science Division) and physicists of DSM (CEA-Matter science Division) specialists of Nuclear Magnetic Resonance (NMR) have developed a new solid state NMR apporach to measure long inter-atomic distances. Based on the use of tritium, the hydrogen isotope with the highest sensitivity to NMR, this technique allows the determination of the conformation of small molecules bounded to their biological receptors. This determination is essential to understand the action mode of these molecules and to design new drugs of simpler structure and easier to synthesize.
NMR offers nowadays many techniques to measure interatomic distances to determine the conformation of one molecule (i.e. the spatial arrangement of its atoms). However, many biological molecules cannot be analyzed by conventional liquid NMR or crystallography because they are insoluble and/or do not crystallize. In that case, solid state NMR has proven to be a valuable approach, but it did not allow, up to now, to measure large distances (typically above 1 nm) between two atoms. The CEA teams have been able to reach such measurements for distances up to ~1.5 nm!
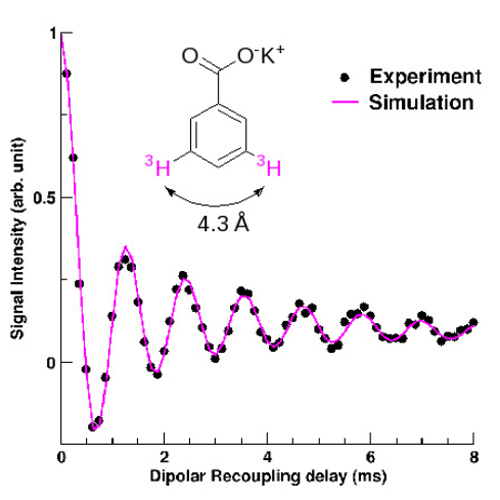
One of the most widespread NMR technique is based on an isotopic labelling of the molecule to be studied, allowing the direct probe of the atoms of interest. The CEA team had the idea of substituting hydrogen atoms for an isotope offering the hignest sensitivity in NMR: 3H tritium (which possess a nucleus with one proton and two neutrons and a nuclear spin of 1/2). They were able to measure by solid-state NMR on a model molecule, a 3H-3H distance of 1.4 nm, well above the 0.5 or 0.6 nm usually obtained. With this new method, currntly extended to biological systems, it will be possible to determine the conformation of macromolecule-ligand systems in relation to their observed biological activity.
Numerous applications of the method are envisaged (ou expected) for the study of products of interest for the pharmaceutical industry, as well as for fundamental researches. An important example is that of paclitaxel (or Taxol). If the action mechanism of this anticancer agent is known for more than 20 years,, the determination of its conformation, when bound to tubulin (a protein involved in the mechanism of cell division) still remains elusive. Improving our knowledge of the Taxol-ligand interaction would have required a technique allowing measurements of distances larger than 1 nm. This is now possible!
Reference :
Measurement of Long-Range Interatomic Distances by Solid-State Tritium-NMR Spectroscopy, A. K. L. Yuen, O. Lafon, T. Charpentier, M. Roy, F. Brunet, P. Berthault, D. Sakellariou, B. Robert, S. Rimsky, F. Pillon, J-C. Cintrat and B. Rousseau, J. Am. Chem. Soc. 132 (6) (2010) 1734.
– Highlight on the DSV website (CEA-Life science Division) (in french)
The isotopic labeling consists in replacing an atom of the molecule you want to study by one of its NMR active isotope (i.e. an atom which nucleus possesses a different number of neutrons, a non zero nuclear spin and with the same chemical properties). Ex: hydrogen replaced by deuterium or tritium.
More informations :