Aluminosilicates and aluminogermanates nanotubes: synthesis and properties
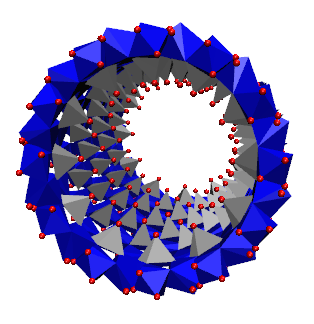
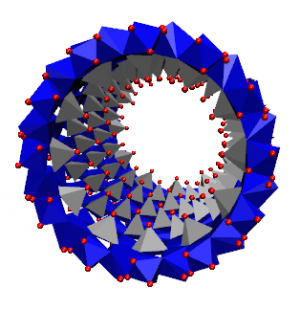
short imogolite nanotube.
Depuis la découverte des nanotubes de carbone (NT), la synthèse et la caractérisation de structures de forme similaire comme les nanotubes inorganiques, les nanotiges ou les nanofils suscitent un grand intérêt. Cependant, des limitations telles que la pureté, la complexité du protocole de synthèse, le faible rendement de production ou la large dispersion de taille restent des obstacles majeurs pour les applications à l'échelle industrielle.
Dans ce contexte, les imogolites synthétiques apparaissent comme une exception. Ce sont des NT d'aluminosilicates à paroi simple de 2 nm de diamètre et jusqu'à 1 μm de longueur, de formule empirique (OH)3Al2O3Si OH, dont la structure a été déterminée par diffraction des rayons X (XRD), résonance magnétique nucléaire (RMN) à l'état solide et microscopie électronique à transmission (MET). Des analogues de l'imogolite, où les atomes de Ge remplacent ceux de Si ont été synthétisés avec succès. Les premiers rapports sur leur synthèse indiquent des conditions très diluées (c'est-à-dire millimolaires). Cependant des analogues d'imogolite ont récemment été obtenus à partir de solutions 100 fois plus concentrées, ouvrant ainsi la voie aux applications à grande échelle. Ces analogues ont été décrits comme étant structurellement identiques à l'imogolite naturelle Al-Si, masi avec un diamètre de tube supérieur (∼3.3 nm) et des longueurs plus courtes (inférieures à 100 nm). Au laboratoire LIONS, le mécanisme de formation de l'imogolite et des analogues de l'imogolite a été largement étudié ainsi que leur utilisation dans un nouveau matériau auto-assemblé.
Since the discovery of carbon nanotubes (NTs), there is great interest in the synthesis and characterization of similar shaped structures like inorganic nanotubes, nanorods, or nanowires. However, limitations such as purity, complexity of the synthesis protocol, low-yield production, and wide size polydispersity still remain major impediments for industrial-scale applications.
In this context, synthetic imogolites appear as an exception. They are singlewalled aluminosilicate NTs of 2 nm diameter and up to 1 μm in length with the empirical formula (OH)3Al2O3Si OH whose structure has been determined using X-ray Diffraction (XRD), solid state Nuclear Magnetic Resonance (NMR), and Transmission Electron Microscopy (TEM). Imogolite analogues where Ge replace Si atoms have been succesfully synthesized. Although early reports of their synthesis involved diluted (i.e., millimolar) conditions, these imogolite analogues were recently obtained from 100 times more concentrated solutions, thereby opening the route for large scale applications. These analogues have been described to be structurally identical to the Al-Si imogolite, except for a larger tube diameter (∼3.3 nm) and shorter length (less than 100 nm). We areng the imogolite and Imogolite analogue formation mechanism. We are also studying the use of Imogolite and Imogolite analogue in self assembled new material.
Contact : Antoine Thill (NIMBE/LIONS)
M. S. Amara, E. Paineau, S. Rouzière, B. Guiose, M.-E. M. Krapf, O. Taché, P. Launois, and A. Thill, Chem. Mater. 27(5), (2015) 1488.
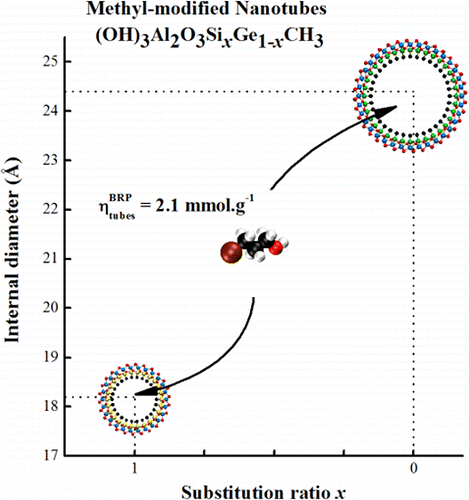
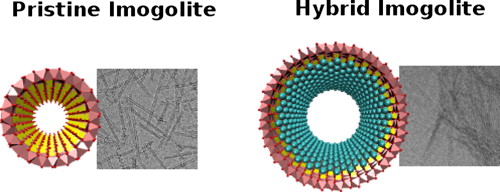
Les développements en nanosciences et les nanotechnologies dépendent fortement de notre capacité à synthétiser de façon bien contrôlée des éléments de taille nanométrique aux propriétés bien spécifiques. Cet article décrit la première synthèse de nanotubes hybrides d'imogolite à paroi simple (OH)3Al2O3SixGe1–xCH3, dont les nanopores hydrophobes ont un diamètre de taille contrôlée entre 1,8 à 2,4 nm. La méthylation et les dimensions des nanotubes sont étudiées en combinant la spectroscopie infrarouge, les observations cryo-TEM et les mesures de diffusion des rayons X. En solution, il est montré que la densité de l'eau à l'intérieur des nanotubes méthylés est réduite d'un facteur 3 par rapport à la valeur initiale. Le confinement spontané des molécules de bromopropanol à l'intérieur des nanotubes, lorsqu'elles sont ajoutées à la solution, est démontré. Il est ainsi montré que ces nanotubes nouvellement synthétisés peuvent ouvrir des possibilités de filtration ou de décontamination de l'eau. Pour en savoir plus…
New developments in nanosciences and nanotechnologies are strongly dependent on our ability to synthesize well-controlled nanobuilding units, with specific properties. We report in this paper the first synthesis of hybrid single-walled imogolite nanotubes (OH)3Al2O3SixGe1–xCH3 with diameter-controlled hydrophobic nanopores varying from 1.8 to 2.4 nm. Methylation and nanotube dimensions are studied by combining infrared spectroscopy, cryo-TEM observations, and X-ray scattering measurements. We show that, in solution, the water density inside methylated nanotubes is decreased by a factor of 3 compared to the bulk value. Spontaneous confinement of bromopropanol molecules inside the nanotubes, when added to the solution, is demonstrated. These newly synthesized nanotubes may open up possibilities for water filtration or water decontamination. learn more…
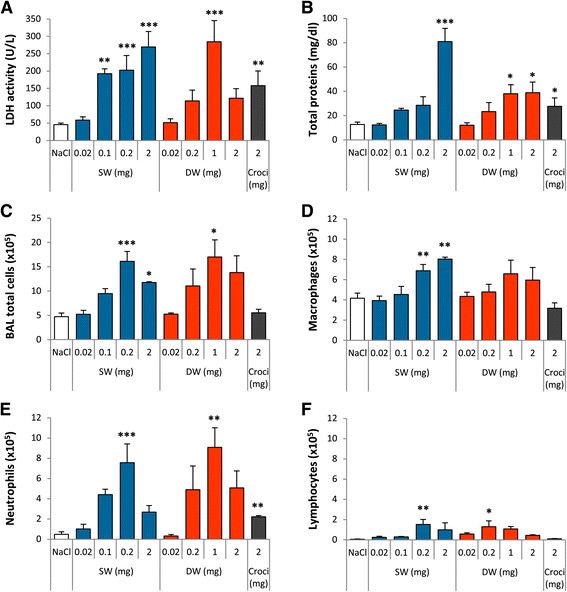
Nanometer-long Ge-imogolite nanotubes cause sustained lung inflammation and fibrosis in rats
S. van den Brule, E. Beckers, P. Chaurand, W. Liu, S. Ibouraadaten, M. Palmai-Pallag, F.Uwambayinema, Y. Yakoub, A. Avellan, C. Levard, V. Haufroid, E. Marbaix, A. Thill, D.Lison & J.Rose, Particle and Fibre Toxicology 11 (2014) 67
Les imogolites-Ge sont de courts nanomatériaux tubulaires d'aluminogermanate dont les potentielles applications industrielles peuvent être intéressantes. Cependant, compte tenu de leurs dimensions nanométriques et de leur rapport d'aspect élevé, il convient d'examiner leur potentiel de toxicité respiratoire. Ici, la biopersistance respiratoire et la toxicité pulmonaire de 2 échantillons de Ge-imogolites de longueur nanométrique sont évaluées. Pour en savoir plus…
Ge-imogolites are short aluminogermanate tubular nanomaterials with attractive prospected industrial applications. In view of their nano-scale dimensions and high aspect ratio, they should be examined for their potential to cause respiratory toxicity. Here, we evaluated the respiratory biopersistence and lung toxicity of 2 samples of nanometer-long Ge-imogolites. Learn more …
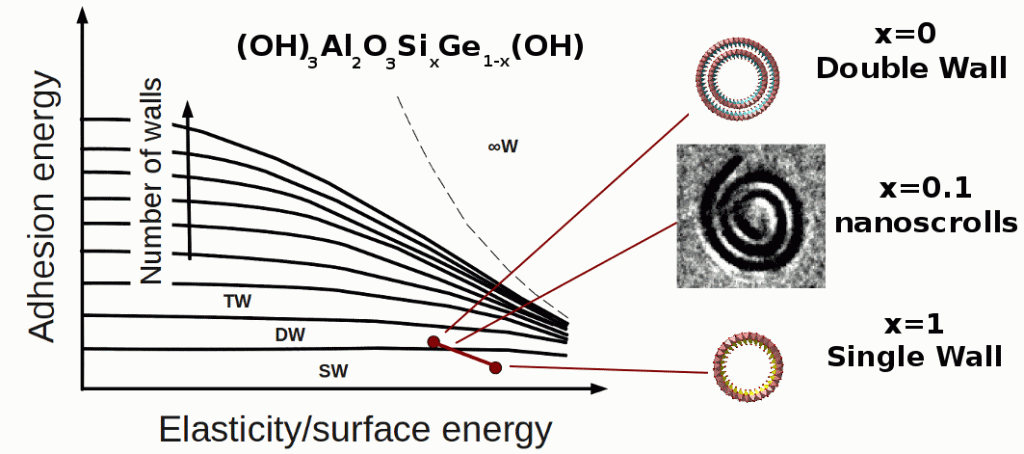
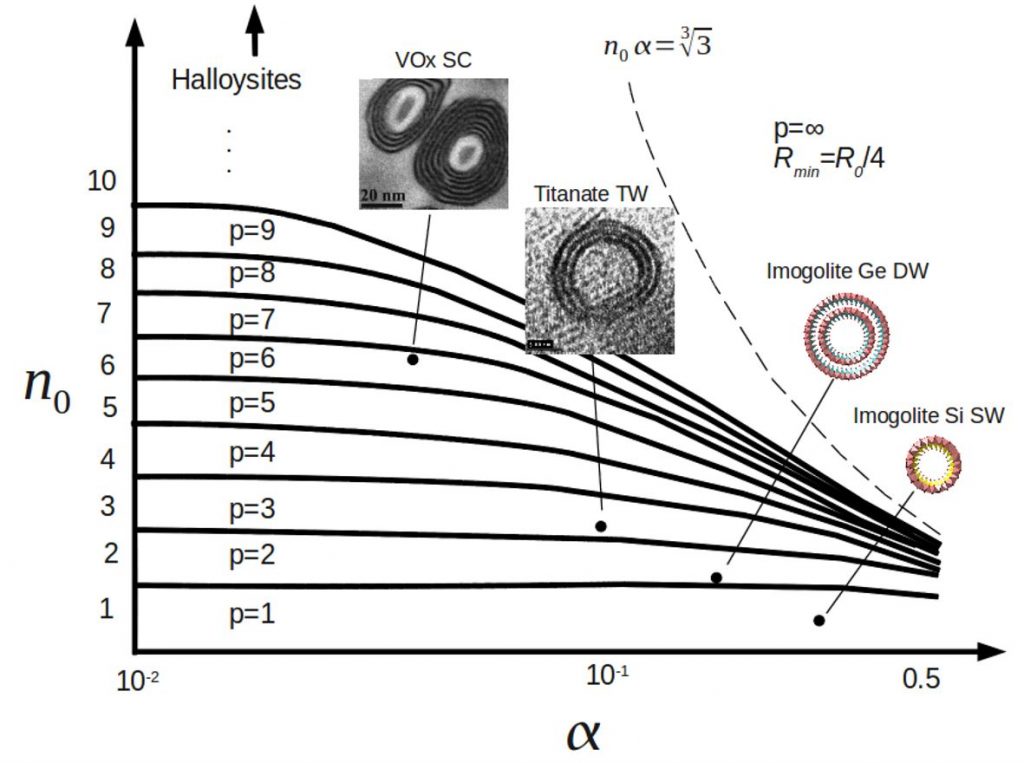
The diameter is controlled by the structure and composition of the nanotube wall and can be tuned by several chemical manipulations. It has recently been discovered that the structure of imogolite nanotubes can change from single-walled (SW) to double-walled (DW) when Si is replaced by Ge during synthesis. Starting from the pure Ge composition, we show that the transition between DW and SW structures can be induced by the incorporation of a small quantity of Si in the synthesis. At that point, the suspension contains a mixture of structures with a nearly constant average diameter. In particular, we found evidence for the presence of a few nanoscrolls. Above 25% Si, SW nanotubes become more stable and present a continuously decreasing diameter with increasing Si. A model is proposed to explain the stability of these different nanotubes and, more generally, the structures of other organic or inorganic nanotubes as a balance between rigidity, surface tension, and adhesion competitive energies.
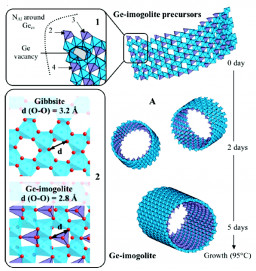
The growth mechanisms of imogolite-like aluminogermanate nanotubes have been examined at various stages of their formation. The accurate determination of the nucleation stage was examined using a combination of local- (XAS at the Ge−K edge and 27Al NMR) and semilocal scale technique (in situ SAXS). For the first time, a model is proposed for the precursors of the nanotubular structure and consist in rooftile-shaped particles, up to 5 nm in size, with ca. 26% of Ge vacancies and varying curvatures. These precursors assemble to form short nanotubes/nanorings observed during the aging process. The final products are most probably obtained by an edge−edge assembly of these short nanotube segments. Learn more…
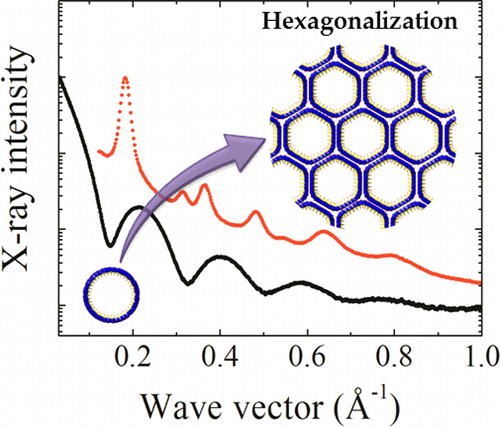
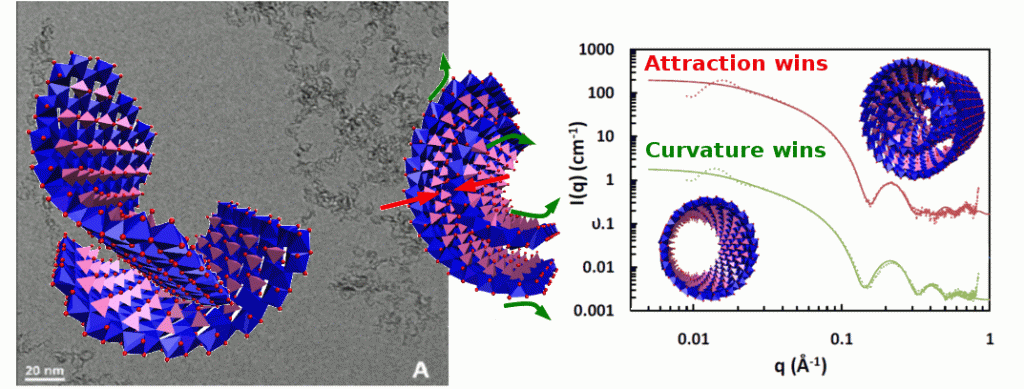
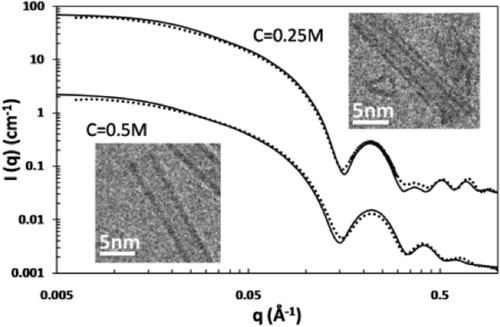
It has been recently discovered that the synthesis of Al−Ge imogolite-like nanotubes is possible at high concentration. Despite this initial success, the structure of these Al−Ge imogolite-like nanotubes remains not completely understood. Using high resolution cryo-TEM and Small Angle X-ray Scattering, we unravel their mesoscale structure in two contrasted situations. On the one hand, Al−Ge imogolite nanotubes synthesized at 0.25 M are double-walled nanotubes of 4.0 ± 0.1 nm with an inner tube of 2.4 ± 0.1 nm. Moreover, SAXS data also suggest that the two concentric tubes have an equal length and identical wall structure. On the other hand, at higher concentration (0.5M), both SAXS and cryo-TEM data confirm the formation of single-walled nanotubes of 3.5 ± 0.15 nm. Infrared spectroscopy confirms the imogolite structure of the tubes. This is the first evidence of any double-walled imogolite or imogolite-like nanotubes likely to renew interest in these materials and associated potential applications.
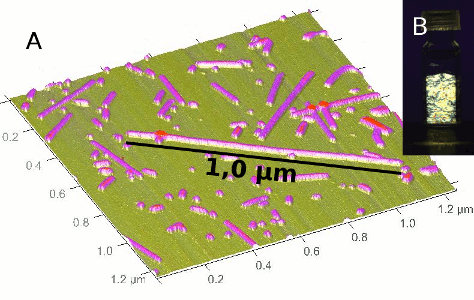
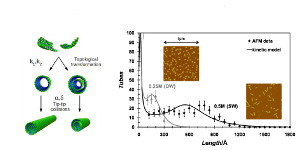
Atomic Force Microscopy (AFM) and in situ Small Angle X-ray Scattering (SAXS) were used to investigate the evolution of the aluminogermanate imogolite-like nanotubes concentration and morphology during their synthesis. In particular, in situ SAXS allowed quantifying the transformation of protoimogolite into nanotubes. The size distribution of the final nanotubes was also assessed after growth by AFM. A particular attention was focused on the determination of the single and double walled nanotube length distributions. We observed that the two nanotube types do not grow with the same kinetic and that their final length distribution was different. A model of protoimogolites oriented aggregation was constructed to account for the experimental growth kinetic and the length distribution differences.
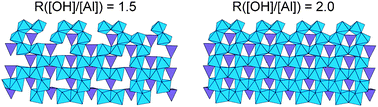
The synthesis protocol for Ge-imogolite (aluminogermanate nanotubes) consists of 3 main steps: base hydrolysis of a solution of aluminum and germanium monomers, stabilization of the suspension and heating at 95 °C. The successful synthesis of these nanotubes was found to be sensitive to the hydrolysis step. The impact of the hydrolysis ratio (from nOH/nAl = 0.5 to 3) on the final product structure was examined using a combination of characterization tools. Thus, key hydrolysis ratios were identified: nOH/nAl = 1.5 for the formation of nanotubes with structural defects, nOH/nAl = 2 for the synthesis of a well crystallized Ge imogolite and nOH/nAl > 2.5 where nanotube formation is hindered. The capability of controlling the degree of the nanotube's crystallinity opens up interesting opportunities in regard to new potential applications.