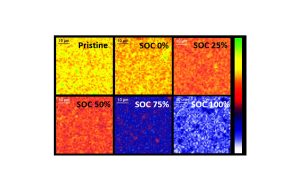
Cells based on LFP cathode materials were extensively analyzed by ex situ and operando IBA at LEEL to understand the local lithium distribution, elemental compositions, etc at different state of charges (SOC).
During the past few years much progress has been made in the development of electrodes and electrolytes for lithium ion batteries, however capacity fading during cycling remains a major problem limiting battery lifetime. This fading phenomenon could be due to the formation of the SEI (Solid Electrolyte Interphase) by electrolyte degradation or by migration of lithium ions at the interface. Several methods have been applied to understand the origins of this loss of capacity. The well-known LiFePO4 cathode material has been the subject of original studies employing a combination of X-ray photoelectron spectroscopy, X-ray diffraction, and X-ray fluorescence. Discriminating FePO4 and LiFePO4 phases indirectly allows local state-of-charge (SOC) evaluation, but none of these aforementioned techniques can display directly the local stoichiometry of lithium.
Conversely, nuclear microprobe is a powerful tool to simultaneously provide qualitative information about the different constituent elements of a positive LiFePO4 electrode present but above all to measure quantitatively their content and distributions as a function of its state of charge (SOC).
The developed method combines the use of two beam conditions (alpha at 2900 keV and protons at 2600 keV) and several analytical techniques (RBS, PIGE and PIXE).
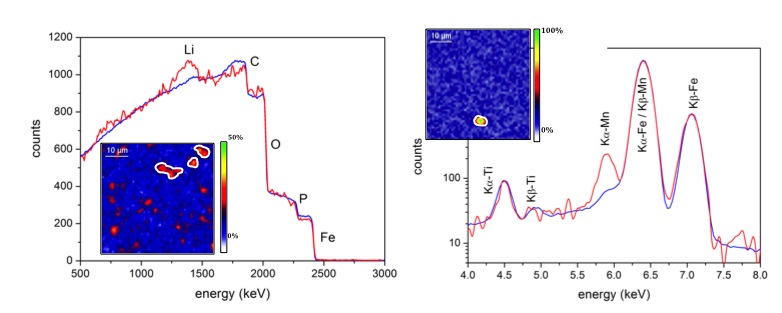
Through ex situ measurements on charged LiFePO4 electrodes, we determined the concentration of light elements such as lithium, and simultaneously the whole composition of the electrode (including majors as well as traces such Mn). We confirmed theoretical compositions at different states of charge (SOC) but a partial immobilization of lithium is observed (Fig. 1), which could be responsible for the loss capacity.
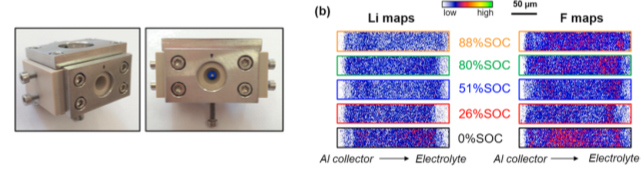
To go further in the characterization of electrochemical cells, we designed an electrochemical cell (Fig. 2 ) to profile operando lithium concentration in LiFePO4 electrodes using Ion Beam Analysis techniques. Direct information about the degradation of the electrolyte is obtained. Particularly we revealed inhomogeneous distributions of lithium and fluorine along the entire thickness of the electrode (Fig. 2 ). Higher concentrations of fluorine are observed near the electrode/electrolyte interface while a depletion of lithium is detected near the current collector at high states of charge.