Automotive electrification and renewable energy storage are currently dominated by Li-ion battery technology, which depends on resources such as lithium, graphite, copper and certain transition metals available in limited quantities and/or unevenly distributed geographically. New battery technologies based on other alkaline or alkaline-earth ions with virtually unlimited resources may in the long term partially replace Li-ion batteries for certain applications. Magnesium-ion batteries are one of these alternative technologies, due to the high abundance of magnesium and the high volumetric and gravimetric capacities that can be achieved.
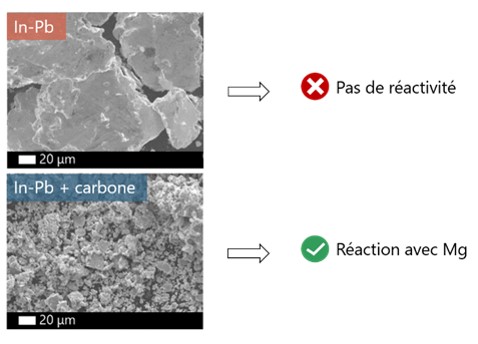
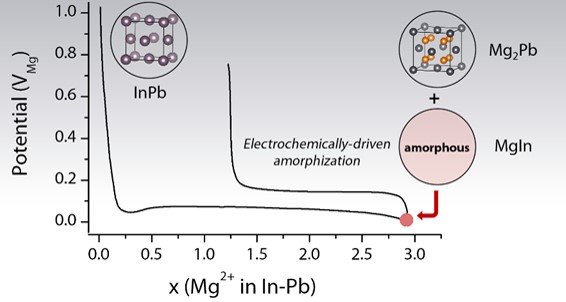
Following on from initial work on the InSb compound, a team at IRAMIS has developed a new negative electrode material for Mg-ion batteries based on the In-Pb compound. The synergistic combination of the electro-active elements In and Pb influences the reaction mechanisms and structure (amorphous/crystalline) of the products formed during the reaction with Mg. This favors high capacitance, but is subsequently detrimental to the material’s reversibility. These results illustrate the influence of electrode amorphization and crystallization processes on the electrochemical performance of batteries.
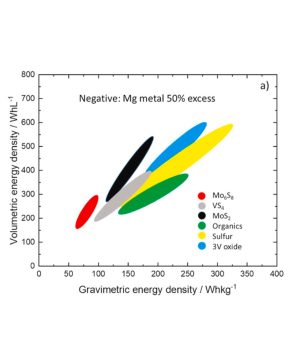
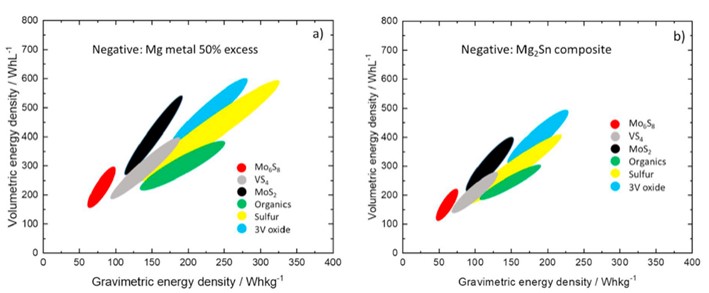
The growth of sectors such as electric vehicles and renewable energy storage is driving battery research, where efforts are now focused on developing more durable, high energy density batteries. One example is the development of magnesium metal (Mg) batteries. Magnesium is emerging as an excellent alternative to lithium, thanks in particular to its high volumetric capacity (3833 mAh/cm3), low cost and abundance in the earth’s crust. For these batteries, the use of metallic magnesium at the anode limits the choice of electrolyte to a few specific compositions, often highly corrosive and with a very narrow potential stability window. On the contrary, electrodes made of certain p-block elements react with magnesium ions in electrolytes with wider stability windows. In a recent perspective review on Mg-ion batteries, contributed by several international groups including NIMBE/LEEL, it is shown that the use of these alloys in place of Mg metal reduces the energy density of the electrochemical cell (see figure below). However, thanks to their greater compatibility with electrolytes and simpler processing, they are potentially interesting anodes for Mg-ion batteries, and synergistic effects are expected for p-block cell combinations.
After proposing an initial alloy based on InSb