D. Kopetzki, Y. Michina, T. Gustavsson, D. Carrière
Amphiphilic molecules have a hydrophilic head and a hydrophobic chain. Under certain conditions, they can self-organize into hollow spherical hollow vesicles with a surfactant bilayer enclosing an aqueous core, the overall diameter ranging from a few tens of nanometers to several microns. By carefully choosing specific preparation conditions, the scientists of SIS2M have shown that extremely robust aggregates can be synthesized with an interesting potential for encapsulation.
The vesicles are widely studied for many fundamental issues (mechanism of self-assembly, physical properties of the membrane, etc …) which understanding may open new perspectives (controlled release of active molecules, chemical nanoreactors, energy conversion, etc…). Usually, these vesicles (in this case also known as “liposomes”) are prepared from phospholipids, that also form cell membranes. It would be advantageous to replace the phospholipids with molecules with similar properties but with more accessible or easily modified chemical functions, such as fatty acids molecules. It was already possible to synthesize such vesicles of fatty acids in specific conditions of temperature and pH, but the way to stabilize them was yet to be found: they are indeed very sensitive to external conditions and are destroyed easily to give micelles or crystals.
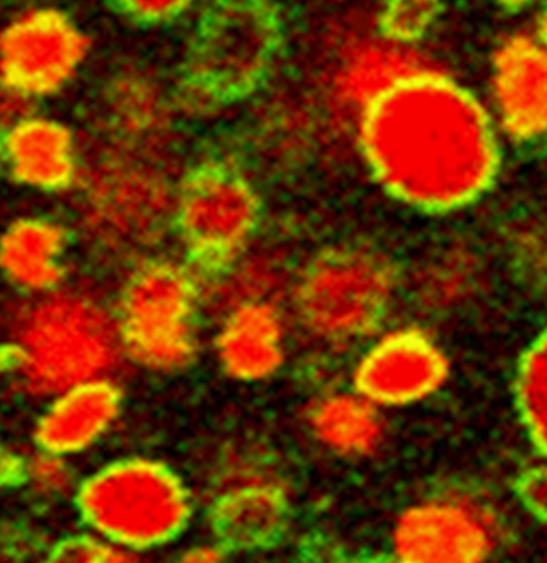
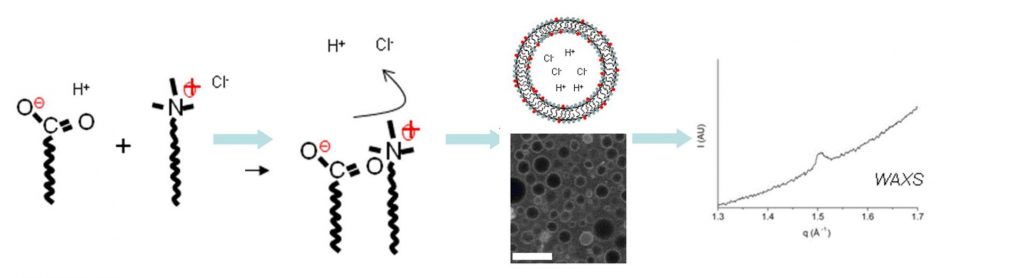
One solution is to design these vesicles in the “gel phase”, that is to say, formed by a solid state surfactant film. Previous studies conducted within SIS2M have shown that in a mixture of fatty acids and cationic surfactants, the mutual attraction between oppositely charged molecules favours the formation of vesicles in the gel phase [1]. This observation has been completed by evidencing the existence of a preferential composition of surfactants for which extremely robust vesicles are formed, that are indestructible by dialysis, dilution, etc. … [2] In these circumstances, the icosahedral porous form, which is usually observed, is no more obtained. The absence of pores then allows encapsulation in a sustainable way of solutes added to the aqueous core of the vesicles, with a performance equal or superior than those of liposomes (Figure 2).
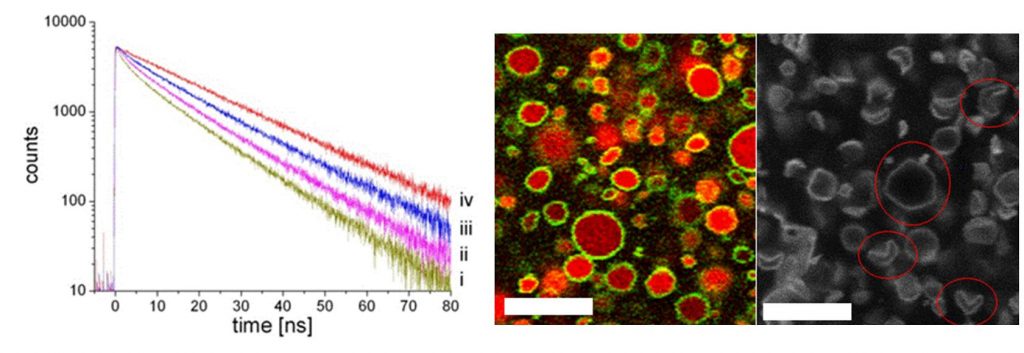
Another original and promising result of the absence of pores is the possibility of “self-encapsulation”: upon association of the two amphiphilic molecules, their respective counter-ions (H+ and Cl–) are released into the solution, and a fraction is trapped into the aggregates [3]. After dialysis, gradients of pH and salt are set between the inside and outside of the aggregates, that are exceptionally durable (several months). This has been demonstrated in experiments using time resolved fluorescence, together with the “Service de Physique des Atomes et des Molécules” (IRAMIS-SPAM). This self-encapsulation of ions allows improvement of our understanding of the morphology of aggregates (flattened aggregates, sphere, icosahedron, concave polyhedron), but also opens unique perspectives related to the control of their size [4]. Indeed, for a vesicle of size L, the number of ions included in the internal volume (~ L3) of the aggregates is proportional to the number of surfactants that form the inner layer (~ L2). The concentration of ions in the aggregates, and therefore all their related properties, is inversely proportional to their size (~ L-1).
In conclusion, vesicles that are robust, impermeable and that show original encapsulation properties have been designed. The mechanisms of stabilization and “self-encapsulation” are very general and have already been extended to functionalized fatty acids or other amphiphilic molecules. Following this approach, these properties have started to be exploited to produce vesicles with more complex properties and applicable to energy conversion.
References:
[1] Self-assembly of regular hollow icosahedra in salt-free catanionic solutions
M. Dubois, B. Deme, T. Gulik-Krzywicki, J. C. Dedieu, C. Vautrin, S. Desert, E. Perez and T. Zemb, Nature, 411 (2001) 672.
[2] Ripening of catanionic aggregates upon dialysis
Y. Michina, D. Carrière, C. Mariet, M. Moskura, P. Berthault, L. Belloni and T. Zemb, Langmuir, 25 (2009) 698.
[3] Fatty acid-cationic surfactant vesicles: counter-ion self-encapsulation
D. Kopetzki, Y. Michina, T. Gustavsson and D. Carrière, Soft Matter, 2009.
[4] G. Béalle, D. Carrière, Size effects and buckling transitions in gel-phase fatty acid vesicles, soumis.





