Cooperation between research teams from the CEA, the CNRS and the Université Paris-Sud[1] has resulted in research showing that chemistry tools subjected to radiation make it possible to study the ageing of electrolytes in lithium-ion accumulators. In particular, accelerated ageing can be produced in electrolytes for the purpose of facilitating studies of their life span. This research was published in Nature Communications on 24 April 2015. Furthermore, the technique can also provide a more accurate understanding of the chemical mechanisms at work in accumulators so as to extend their life span and make them safer to use.
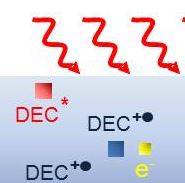
Over the course of the lifetime of a set of accumulators, the electrolytes that enable the transporting of ions that store energy in chemical form are subject to deterioration. This limits the length of time during which the equipment can be used. The electrolyte is therefore a key component for ensuring safe, reliable and powerful batteries in mobile applications (mobile phones), cars (power batteries) and installations on electrical circuits (a). In the automotive industry, in particular, hybrid and electrically-powered vehicles require particularly efficient reversible energy storage due to the power used in the large number of charging-discharging cycles.
Scientists are therefore using various means by which to study the chemical processes that cause electrolytes to deteriorate as they age. Studies of ageing through repeated charging and discharging cycles of the chemical process causes an electrolyte to deteriorate as it ages. Studies of ageing through repeated charging and discharging cycles, however, are lengthy and costly.
Faster studies
The three-pronged CEA-CNRS-Université Paris-Sud cooperation initiative became especially interested lithium-ion batteries since these are to be found in all portable electronic systems, including vehicles (motor-bikes, cars, etc.) or in the management of energy networks powered by intermittent sources (b).
“To accelerate the ageing process, we subjected electrolytes to ionising radiation by using ‘irradiated chemicals’ or radiolysis tools”, explains Sophie Le Caër (a CNRS researcher at the NIMBE laboratory) who is running the study. The great advantage of radiolysis is that it produces controlled ageing of the electrolyte within a few minutes or a few hours while, by electrically performing battery charging and discharging cycles, it can take weeks or even months to obtain the same effects. “We irradiated diethylcarbonate, a solvent typical of those used in the electrolytes of lithium-ion accumulators”, continued Sophie Le Caër. “We observed the formation of the same molecules as occurred after electrolysis”. An electrolyte consists of a salt in a solvent or mixture of solvents. Irradiation of the chemical makes it possible to study the solvent specifically with or without the salt, and thus to acquire a detailed understanding of the reactivity of each part of the system.
Monitoring the dynamics of the deterioration of the electrolyte step-by-step
In addition to this demonstration published in Nature Communications, radiolysis, combined with effective chemical analysis techniques, makes it possible to reveal the formation of molecules, even minor ones. “this enables us to gain a more precise understanding of the system and highlight the mechanisms that eventually contribute to a reduction in battery life”, explains Sophie Le Caër. “Furthermore, studies can be made of how the system changes over time and make it possible to monitor the system for very long periods of time, ranging from one picosecond, the measurement of local chemical reaction, to up to one day (17 times as long!). This combined research thus opens the way to performing chemical experiments under radiation to sift through the electrolytes and identify those that are best for use in accumulators in the future”.
Reference for the article:
|
Radiolysis as a solution for accelerated ageing studies of electrolytes in Lithium-ion batteries, D. Ortiz, V. Steinmetz, D. Durand, S. Legand, V. Dauvois, P. Maître, S. Le Caër, Nat. Comm., 6 (2015) 6950. |
 |
Other references:
(a) “Towards greener and more sustainable batteries for electrical energy storage”,
D.Larcher, J. M. Tarascon, Nature Chem. 7, 19 (2015). http://dx.doi.org/10.1038/nchem.2085
(b) “Issues and challenges facing rechargeable lithium batteries”,
J. M. Tarascon, M. Armand, Nature 414, 359 (2001).
[1] Laboratoire de chimie-physique, (CNRS/University of Paris-Sud), Direction de l’énergie nucléaire of the CEA (CEA DEN) and the laboratoire Nanosciences et Innovation pour les Matériaux, la Biomédecine et l'Energie (NIMBE – CEA IRAMIS/CNRS)
CEA-CNRS press release: Batteries Li-ion : le vieillissement des accumulateurs étudié grâce à la chimie sous rayonnements ionisants.
Contact CEA-IRAMIS : Sophie Le Caer and Daniel Ortiz, Institut Rayonnement Matière de Saclay – NIMBE UMR 3685 CEA‐CNRS.
Collaborations:
- CEA/Saclay, DEN/DANS/DPC/SECR/LRMO F-91191, Gif-sur-Yvette Cedex, France.
- Laboratoire de Chimie Physique, UMR 8000 CNRS-Université de Paris Sud, 91405 Orsay Cedex, France
- ICMMO UMR 8182, CNRS-Université de Paris Sud, 91405 Orsay Cedex, France
- CEA/Grenoble, LITEN/DEHT/SCGE, 38000 Grenoble, France.

