M. Roger1, D.J.P Morris2, D.A. Tennant3,4, M.J. Gutmann5, J.P. Goff2, J.-U. Hoffmann3, R. Feyerherm3, E. Dudzik3, D. Prabhakaran6, A.T. Boothroyd6, N. Shannon7, B. Lake3,4 & P.P. Deen8.
1Service de Physique de l’Etat Condensé (CNRS/MIPPU/URA 2464), DSM/DRECAM/SPEC, CEA Saclay, P.C. 135, F-91191 Gif sur Yvette, France.
2Dept. of Physics, University of Liverpool, Oliver Lodge Laboratory, Liverpool L69 7ZE, UK.
3Hahn-Meitner Institute, Glienicker Strasse 100, Berlin D-14109 Germany.
4Institute für Festkörperphysik, Technische Universität Berlin, Hardenbergstrasse 36, Berlin D-10623 Germany,
5ISIS Facility, Rutherford Appleton Laboratory, Chilton, Didcot, Oxon OX11 OQX, UK.
6Clarendon Laboratory, Parks Road, Oxford OX1 3PU, UK.
7H.H Wills Physics Laboratory, University of Bristol, Bristol BS8 1TL, UK.
8European Synchrotron Radiation Facility, BP 220, F-38043 Grenoble Cedex, France.
Cobaltates are ABX type compounds where B indicates cobalt or an adjacent atom in the Mendeleïev table. They are of great technological interest because of their thermoelectric properties.
These properties crucially depend on the structure. Cobaltates AxCoO2 exhibit successive layers of cobalt oxide CoO2. The variable proportion x of alkaline ions fits between these layers. This makes possible to store and restore electric charges. Thus, Li based compounds (A=Li) have appeared a few years ago in the batteries of our cell phones.
The adjustment of the density of conducting electrons within the cobalt plans, allows controlling the electric and magnetic properties o the material. This situation is similar to that of the intensively studied case of high critical temperature superconductors, but with two main differences:
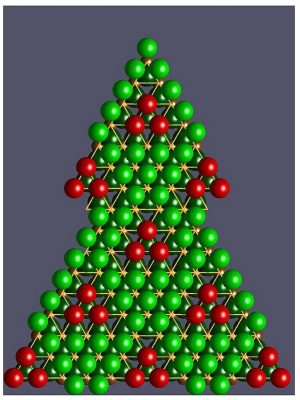
i) Contrary to square networks of cuprates, the cobalt atoms are ordered according to a triangular lattice (see figure).
ii) The sodium vacancies in NaxCoO2 are not distributed randomly, but are ordered according to a nanometric superstructure which reinforces the periodic potential seen by the mobile electrons of the underlying cobalt plans.
This second point is the subject of a new paper in the journal Nature, by a European team of scientists lead by a physicist of the “Service de Physique de l’Etat Condensé”, CEA-Saclay, and researchers from the University of Liverpool and the Hahn Meitner Institute in Berlin.
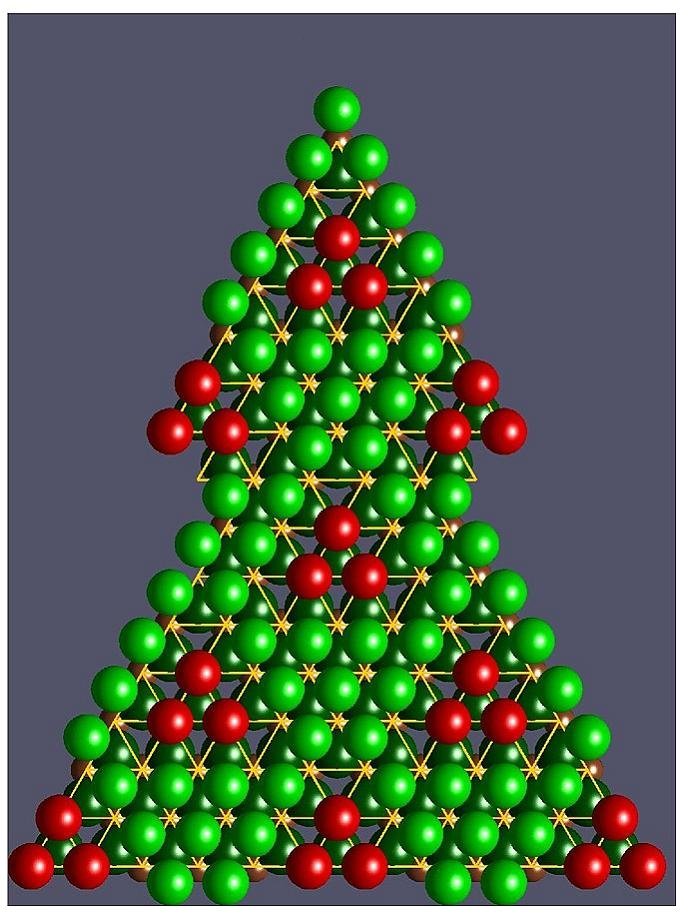
For the sodium saturated compound NaCoO2 (x=1), the network of sodium ions forms a compact stacking of spheres (green on the figure) projecting itself in the center of the cobalt triangles. For x < 1 the sodium vacancies order in a periodic structure whose mesh is about 5 times the inter-cobalt distance (~ 1 nanometer, see figure for Na0.8CoO2). In this structure, the position of some sodium atoms (red) gathered in triangular clusters projects on the nodes of the network of cobalt atoms. They are separated from the atoms in centered position (green) by three vacancies along the edges of the red triangle.
– For the electric conduction, these triangular clusters (red) exert important repulsive electrostatic forces on the mobile charges of the underlying plans of cobalt which are thus channeled. According to the proportion of sodium ions (x), the conduction can be two-dimensional (Na0.8CoO2) or one-dimensional (Na0.5CoO2). In all cases, interactions between mobile charge carriers and their “effective mass” are strongly enhanced.
– Concerning atomic vibrations, the “triangular cages of vacancies” give more freedom to the sodium atoms of each cluster, enabling them to absorb a high proportion of any heat traveling through the compound. This explains the low thermal conductivity observed.
These properties induce the strong thermoelectric power of these materials, which appear as good candidates in technological applications (cooling of electronic devices or –reciprocally- conversion of wasted heat into electricity). Moreover, their very rich transport and magnetic properties are tunable through the sodium content.
These conclusions are the result of remarkable neutron diffraction experiments performed at the ISIS pulsed source (Rutherford Appleton Laboratory, U.K.) and at the Institute Hahn Meitner of Berlin, confronted with a simple model where electrostatic interactions and steric stacking constraints are primarily taken into account. Excellent mono-crystals were obtained at the Clarendon laboratory of Oxford by the “floating zone technique”. Numerical calculations were executed in Saclay and on the massively-parallel computer MAP2 of Liverpool University.
Bibliography:
M. Roger, D.J.P Morris, D.A. Tennant, M.J. Gutmann, J.P. Goff, J.-U. Hoffmann, R. Feyerherm, E. Dudzik, D. Prabhakaran, A.T. Boothroyd, N. Shannon, B. Lake and P.P. Deen,
Patterning of sodium ions and the control of electrons in sodium cobaltate
Nature 445 (2007) 631. Voir aussi : cond-mat/0507040.
07-02-2007