Principles and applications
Principle of laser pyrolysis
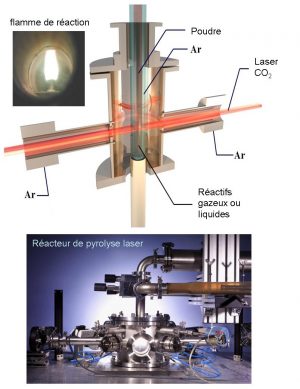
It’s based on cross-jet interaction between aCO2 infrared laser beam and a stream of reactants in a reactor under controlled atmosphere. The transfer of energy causes a rise in temperature in the reaction zone, the precursors are dissociated, and a flame appears in which nanoparticles are formed without interacting with the reactor walls. Precursors can be gaseous or liquid. In the case of a liquid, the precursor is injected into the reactor as an aerosol.
Among the various methods for synthesizing nano-objects, laser pyrolysis stands out for its flexibility and the variety of compounds it can produce in terms of chemical composition, morphology and crystallinity.
The steps in the process are
- Excitation of the vibrational states of molecules absorbing infrared radiation
- Transfer of excitation by collision to all molecules in the medium
- Dissociation of the molecules, giving rise to a saturated vapor
- Homogeneous nucleation
- Nanoparticle growth
Chemical yields can exceed 90% for gaseous precursors.
Production rates range from 30 to 100 g/h for Si-based nanopowders in the laboratory, and up to 1 kg/h on a production pilot for SiC nanoparticles.
The main adjustable parameters are :
- The nature of the precursors (e.g. SiH4, C2H4, HMDS, TEOS, etc.)
Thanks to this wide variety of precursors, various nanostructured powders have already been obtained by laser pyrolysis, for example: Si, SiC, Si/C/N, Si/C/N/Y/Al, Si/C/B/N, Si/C/O, a-C:H, C, C60, C70, C-N, B4C, TiC, WC, FeC, Fe3C, TiB2, ZrB2, Fe4N, Fe, FeO, Fe2O3, TiO2, Al2O3, V2O5, CrO2, etc. - Laser power
In particular, it enables us to control the flame temperature and therefore the crystallinity of the products. - Reagent flow
This governs the residence time of the reagents in the flame. It thus enables nanoparticle size to be adjusted over a wide range.
A wide range of applications
Example 1: Nanopowder oxides for catalysis.
The fight against pollution includes research into the destruction of Volatile Organic Compounds (VOCs). In this context, the need for more efficient catalysts for air and water treatment is growing. Nanopowders synthesized by laser pyrolysis show very promising properties.
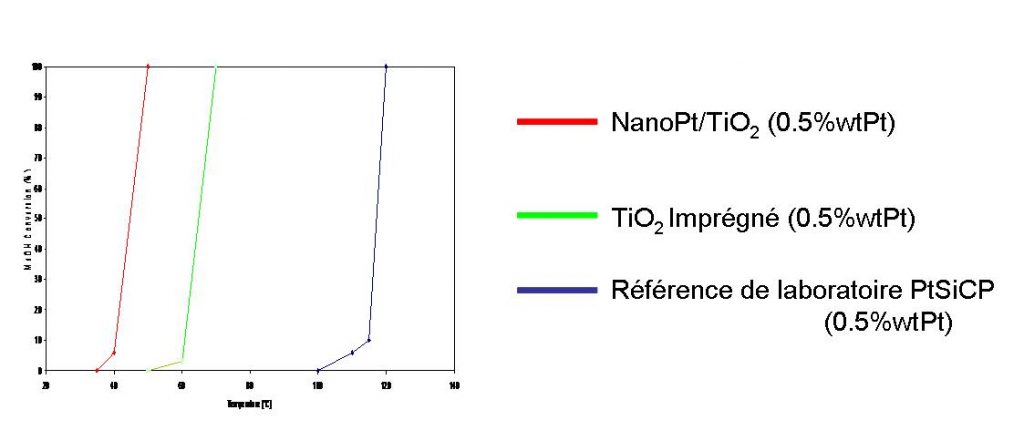
The synthesis of TiO2 nanoparticles and Pt nanocomposites on TiO2 has been carried out in the laboratory. The precursors are : Ti(OC3H7)4 Pt acetyl acetonate (aerosol) C2H4 (sensitizer). At the reactor outlet, TiOxCy Pt(x ~ 2) powders are produced with concentrations that can be adjusted down to 5% Pt. Heat treatment in air at 400°C removes the carbon.

In collaboration with the Laboratoire de Catalyse en Chimie Organique in Poitiers, tests were carried out on the catalytic properties of the nanoparticles using methanol oxidation. These studies show that Pt-TiO2 synthesized by laser pyrolysis is active from 45°C, whereas conventional Pt-TiO2 obtained by impregnation is only active from 65°C.
Example 2: Nanostructured carbide ceramics for4th generation nuclear reactors.
The aim of this research is to develop and study new materials that meet the requirements of the nuclear industry of the future, particularly in terms of mechanical strength under high-temperature irradiation. In this context, nanostructured carbide materials (SiC, ZrC, TiC) appear to be interesting candidates. They offer improved mechanical properties compared with conventional ceramics, and thanks to their high grain boundary density, could prove more resistant to irradiation damage.
The synthesis of TiC nanopowders is initiated by precursors of the Ti(OC3H7)4 (aerosol) C2H4 (sensitizer) type.
At the reactor outlet, TiOxCy(x ~ 2) powders are formed.
Annealing under Argon enables the formation of nanostructured TiC.
Nanostructured ZrC is synthesized using a similar method.
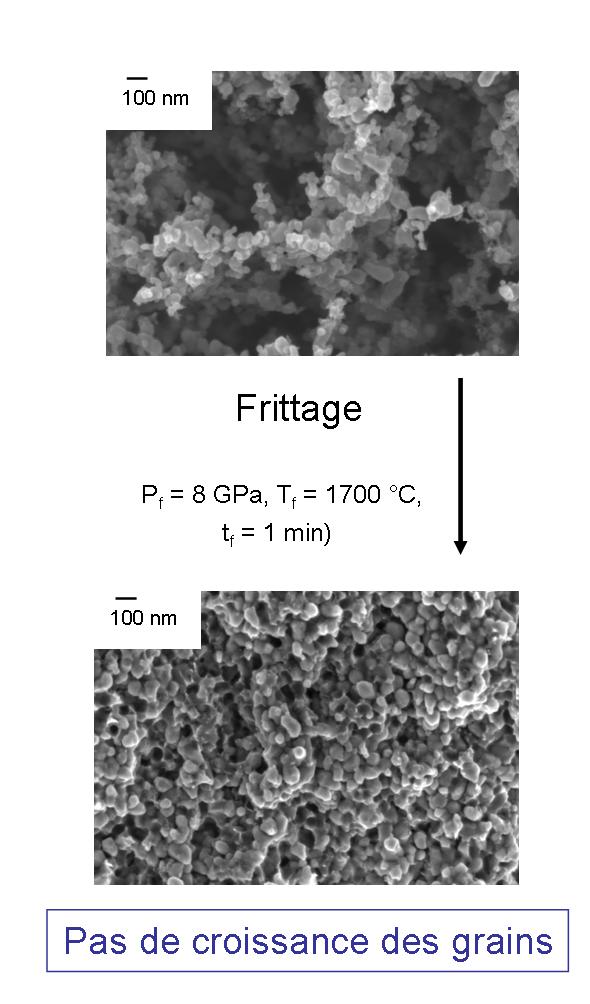
The development of nanostructured ceramics and the study of their properties are carried out in collaboration with the Institute of High Pressures in Warsaw, which has developed a flash sintering technique particularly suited to nanomaterials.
As a result, no nanograin growth is observed during the sintering process.
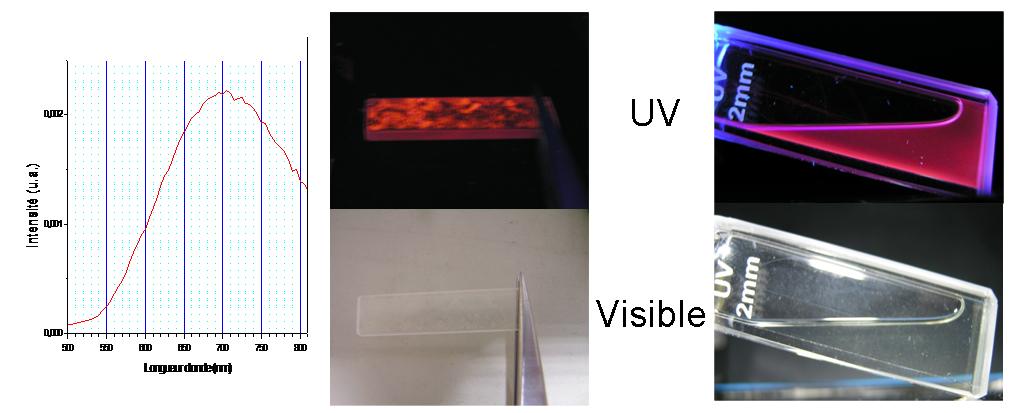
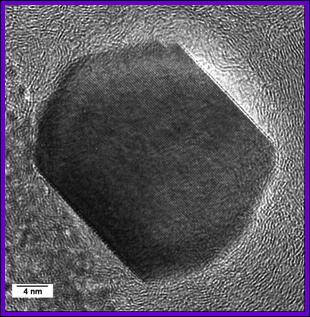
Example 3: Silicon nanocrystals for biocompatible optical fibers and photoluminescent markers
Unlike bulk Si, which emits in the infrared under UV radiation, silicon nanocrystals show visible photoluminescence when their size falls below 10 nm. This property is attributed to the quantum confinement effect.
Research into the synthesis of these nanocrystals focuses on the study of experimental parameters (dilution, reactor pressure, laser power, etc.). The synthesis of nanocrystals in weightable quantities down to 4 nm has been developed in the laboratory.
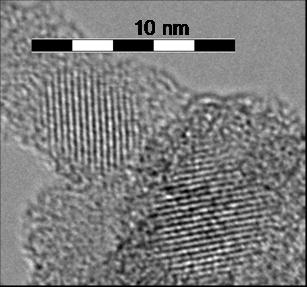
Optical properties are studied in collaboration with the Laboratoire de Physico-chimie des Matériaux Luminescents in Lyon.