Nuclear Magnetic Resonance (NMR) spectroscopy is a powerful tool for biology, allowing structural and chemical analysis of metabolites as well as imagery (MRI). A collaboration of scientists from Nimbe, Neurospin and Bordeaux University, has recently designed a non-invasive online NMR μ-probe for profiling in-vivo metabolic physiological activities with a micro-size NMR detector placed in “close proximity” to a microdialysis sampling probe. Such a device is able to perform real-time diagnostic, deciphering complex metabolic activities.
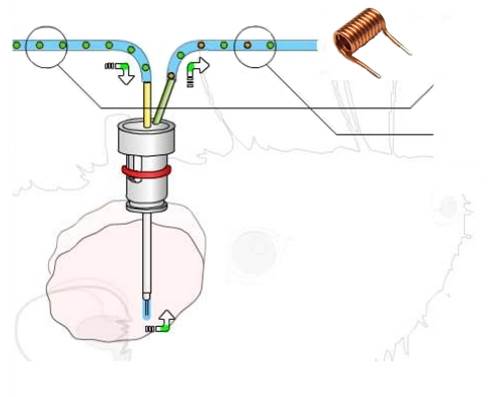
The “chemical” monitoring of the functioning of a biological cell requires the analysis of its metabolites, i.e. the products of its “digestion” of the various available nutrients . NMR is one of the best tools for this analysis because it makes it possible to distinguish metabolites and their manufacturing mechanism, in particular by the isotopic enrichment of the nutrients.
With its limited invasiveness, microdialysis is a commonly used sampling technique for in vivo investigations of biochemical functions in animals and in patients for severe diseases. It is often remotely coupled with an analytical technique like chromatography, mass spectroscopy or NMR spectroscopy, for metabolic analyses. In practice, a fine needle-like probe is inserted into the body tissue, and a solution – perfusate, similar to the tissue fluid – is continuously injected. At the outlet, the fluid (dialysate) contains the biochemicals (metabolites), in response to the stimuli, which can be simultaneously collected for remote analysis.
However, the sampling volume in microdialysis is very limited (a few μL), making nearly impossible a real-time analysis of the dialysate with NMR spectroscopy. The team at ISM (Université de Bordeaux) and Alan Wong at LSDRM (CEA-Saclay) have taken the challenge to implement an online NMR μ-probe for a rapid profiling of the metabolite compositions in dialysate [1]. For this purpose, a micro-size NMR detection coil is positioned at the immediate outlet of the microdialysis. This allows enhancing the detection sensitivity and offering the possibility of real-time acquisitions of NMR spectra of the dialysate. Moreover, the probe is entirely set inside a MRI scanner, allowing for subsequent acquisition of MRI images for assessment of the microdialysis products.
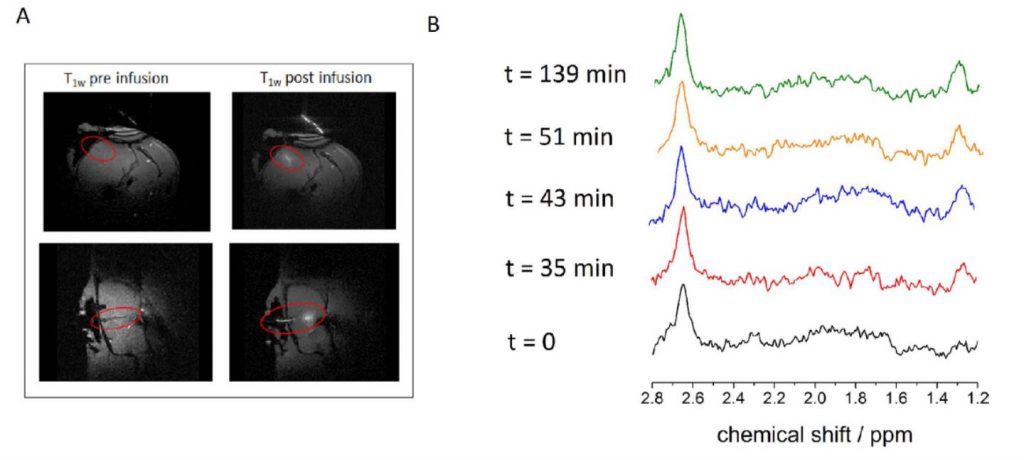
As a proof-of-concept, the NMR μ-probe was tested on in vitro tumor cells and in vivo animal brain, with an NMR sampling volume of about 1 μL. The acquired online NMR spectra (in less than 4 minutes for each acquisition) clearly show an increasing level of extracellular lactate – a known biomarker for an active cerebral metabolism [2] or tumor aggression. By comparing the signal intensity with an internal reference, an equilibrium lactate concentration of about 1 mM is measured.
The current setup is not yet optimum; the detection µ-coil could be further improved for faster real-time detection and better analytical sensitivity. Nonetheless, the proposed online μ-probe is the first-step towards a clinical NMR tool, allowing an effective approach of microdialysis with patients suffering of disorders affecting various organs, such as a breast tumor, liver, adipose tissue or skeletal muscles [3,4]. Analyzes by this type of NMR μ-probe will be essentially integrated in medicine for real-time diagnosis and better prognoses.
References:
- In vivo online magnetic resonance quantification of absolute metabolite concentrations in microdialysat
Stefan Glöggler, Silvia Rizzitelli, Noël Pinaud, Gérard Raffard, Vanessa Zhendre, Véronique Bouchaud, Stéphane Sanchez, Guillaume Radecki, Luisa Ciobanu, Alan Wong, Yannick Crémillieux, Scientific Reports 6, 36080 (2016).

- ‘Glucose and lactate metabolism in the awake and stimulated rat: a 13C-NMR study’
D Sampol, E Ostrofet, M.L.. Jobin, G. Raffard , S. Sanchez , V. Bouchaud , J.-M. Franconi , G. Bonvento , A.-K. Bouzier-Sore
Frontiers in Neuroenergetics 5, 5 (2013). - ‘Soft tissue penetration of cefuroxime determined by clinical microdialysis in morbidly obese patients undergoing
abdominal surgery’
A. Barbour, S; Schmidt, W. Robert Rout, K. Ben-David, O. Burkhardt, H. Derendorf, International Journal of Antimicrobial Agents, 34, 231 (2009). - ‘Cerebral microdialysis in clinical studies of drugs:
pharmacokinetics applications’
RJ Shannon, KLH Carpenter, MR Guilfoyle, A Helmy, PJ Hutchinson, . Journal of Pharmacokinetics and Pharmacodynamics, 40, 343 (2013).
Collaboration:
- Alan Wong at NIMBE/LSDRM
- Yannick Crémillieux, ISM, UMR 5255, Université Bordeaux, 33076, Bordeaux, France
- Luisa Ciobanu, CEA I2BM NeuroSpin, 91191, Gif-sur-Yvette, France
This work is supported by Laboratory of Excellence TRAIL ANR-10-LABX-57 (research program Oncoflux).
Contact CEA-IRAMIS: Alan Wong (NIMBE/LSDRM)




