Our research aims to provide innovative responses in the field of the environment to meet societal challenges.
The main applications targeted are the development of innovative pollution control treatments and the detection of toxic species in aqueous media. For this, our fundamental research activities, in connection with electrochemistry, and with application aims, revolve around the following axes:
Depollution: Innovative treatments according to two strategies depending on the pollutants targeted (Sophie Peulon)
Strategy 1: “Copy and Optimize Nature”
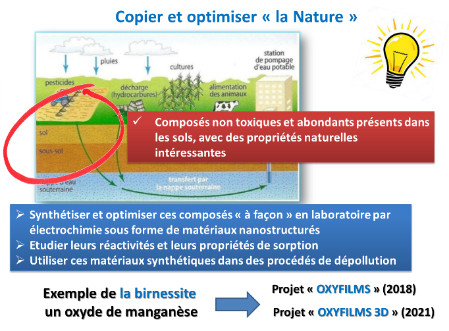
In soils, there are non-toxic and abundant compounds that exhibit interesting natural properties. The main idea is therefore to synthesize and optimize them “custom” by electrochemistry in the form of nanostructured thin films.
Indeed, electrodeposition is a perfectly adapted method to synthesize a multitude of pure compounds in the form of thin, adherent and homogeneous films, with easily modulable nanostructures. In addition, the syntheses are rapid, take place at room temperature, with low energy requirements, and very good reproducibility.
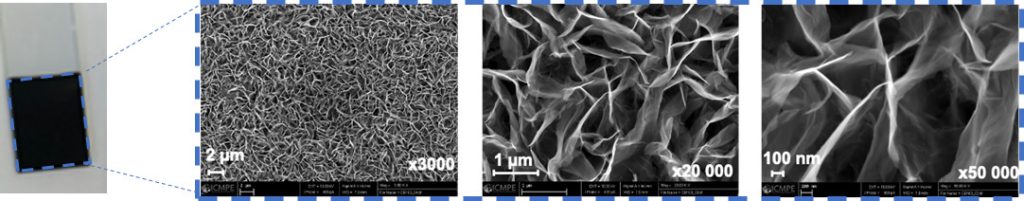
Example of an electrodeposited birnessite film (Scanning Electron Microscope)
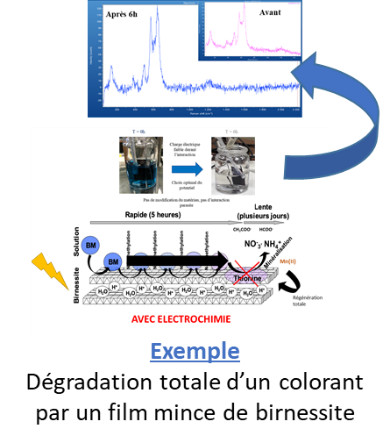
These materials are then used to spontaneously degrade organic pollutants to mineralization (redox reactions), with partial self-regeneration capabilities, or to adsorb inorganic pollutants (heavy metals). They can also be used as electrode materials, which allows, with reduced energy inputs, a significant amplification of the degradation capacities, of the kinetics, in addition to a total regeneration of the material.
Associated references :
- Publications
- M. Zaied, S. Peulon et al., Appl. Catal. B: Environ. 2011, 101, 441.
- M. Zaied, S. Peulon et al., Appl. Catal. B: Environ. 2011, 107, 42.
- M. Ndjeri, S. Peulon et al., Colloids Surf. A 2013, 435, 154.
- A. Pensel, S. Peulon, Electrochem. Com. 2016, 69, 19.
- R. Choumane & S. Peulon, Coll. Surf. A, 2019, 577, 594.
- F. Tsin, A-M Gonçalvès, S. Peulon, et al. Appl. Surf. Science 2020, 525, 146555.
- C. Boillereau & S. Peulon, J. Water Process Eng., 54, 2023, 104024.
- Patents
- A. Pensel, S. Peulon, CNRS Patent WO/2022/018383, 2022.
- PhD Theses
- M. Zaied, Université d’Evry Val d’Essonne, cotutelle with Tunisia, 2012.
- M. Ndjeri, Université d’Evry Val d’Essonne, 2012.
- A. Pensel, 2016, Université d’Evry Val d’Essonne.
- R. Choumane, 2018, Université d’Evry Val d’Essonne
- C. Boillereau, Université Paris-Saclay, 2023.
- Project
- Maturation SATT Paris-Saclay « Oxyfilms » (2024)
Strategy 2: “Eliminate, Separate & Valorize”
Among the many pollutants encountered, heavy metals are particularly problematic due to their high toxicity, their total absence of (bio)degradation, and their accumulation in the environment, via fauna and flora, then in the food chain with important consequences for humans. The paradox is that these same heavy metals are resources in tension, because they enter into the composition of materials intended for the “energy transition”, and that their exploitation has dramatic consequences on the environment. There are processes for purifying polluted water, but their performance must be constantly improved due to the revision of standards, while reducing costs. In addition, these methods do not make it possible to easily separate the heavy metals from each other due to their chemical properties, and as a result they are recovered in a non-valorizable form.
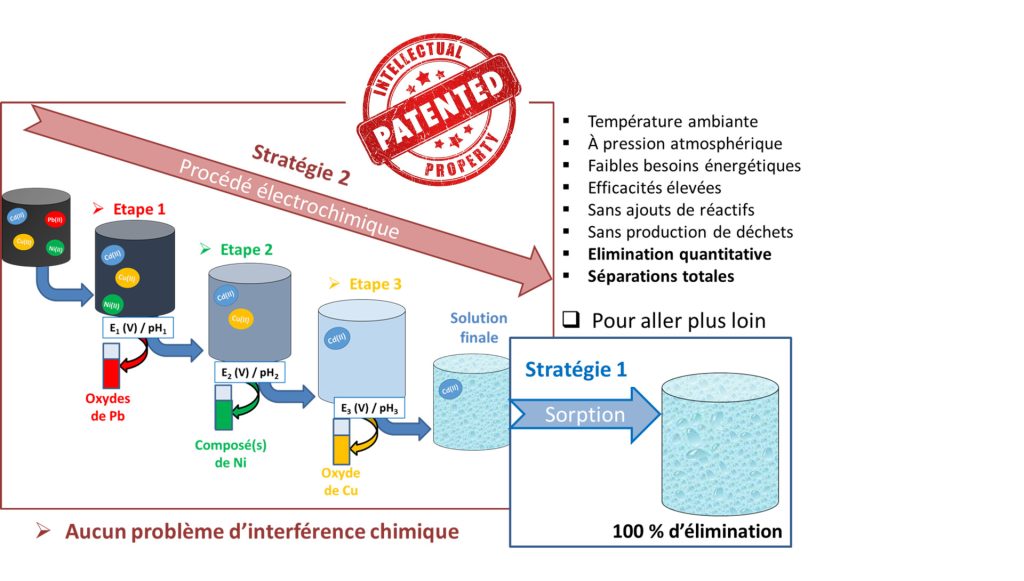
It is in this context that we are interested in developing innovative depollution processes with regard to heavy metals. The recently developed strategy is based on the original idea of eliminating them by electrochemical techniques in the form of adherent non-metallic thin films. Thus, this process is very effective in eliminating lead, for example, up to 99.99%, making it possible to directly reach concentrations acceptable for the environment and drinking water according to the standards set by the World Health Organization. (WHO).

General principle of depollution of an aqueous solution loaded with heavy metals
This process, very simple to implement at low energy costs, also makes it possible to easily separate several heavy metals initially contained in the same solution (for example lead, nickel and copper), and to recover them each in the form of adherent films, which could be interesting for many applications ((electro)catalysis, depollution, energy). In view of all these advantages, this process has been patented (French, international extensions), and published.
Schematic illustration of the depollution of an aqueous solution loaded with heavy metals by coupling the two complementary strategies
In the case of a mixture containing cadmium, since it cannot be eliminated by this electrochemical process due to its own chemistry, it could be eliminated with the materials developed by strategy 1 (films of manganese oxides) as illustrated above, thus allowing total depollution of the aqueous solution, with separation of all the heavy metals initially present in solution.
These studies are therefore extremely promising for developing innovative depollution processes (eliminate & separate) but also for the valorization of the materials obtained in the context of a circular economy (recycling). Indeed, these present completely original morphologies and never reported in the literature because of very specific conditions of obtaining.
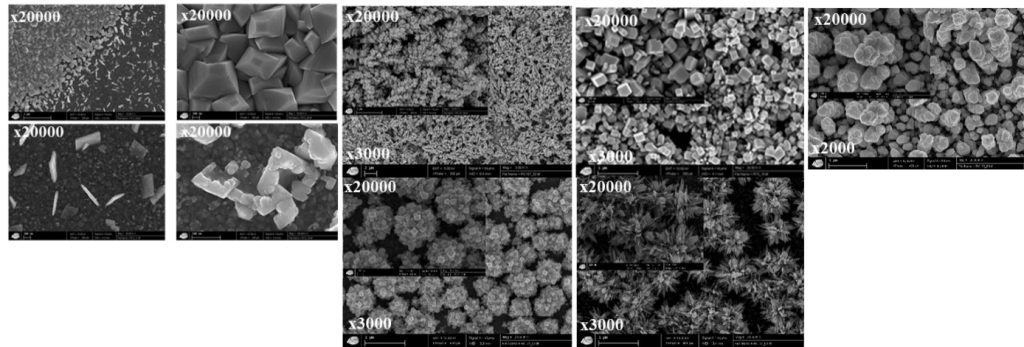
Examples of morphologies obtained for the same lead oxide depending on the conditions
It is in this context and this dynamic that a CFR thesis began in October 2022 with two main axes, namely the optimization of the process for depolluting wastewater and the possibility of recovering the materials obtained.
Associated references :
- Publications
- R. Choumane, S.Peulon, Chem. Eng. J 2021, 423, 130161.
- R. Choumane, S.Peulon, J. Environ. Chem. Eng. 2022, 10(6), 108607.
- R. Choumane, S.Peulon, Journal of Water Process Engineering, 54, 2023, 103900.
- Patents :
- S. Peulon, R. Choumane, CNRS Patent BFF 18P0262 SGA, 2018.
- S. Peulon, R. Choumane, CNRS Patent PCT/EP2019/067482 28/0619, 2019.
- PhD Theses :
- R. Choumane, 2018, Université d’Evry Val d’Essonne.
Detection: Development of innovative electrochemical devices for the analysis of pollutants based on liquid-liquid microinterfaces (Caroline Cannizzo & Sophie Peulon)
The main originality of these devices is the possibility of detecting charged species, by direct or indirect transfer via the use of selective complexing agents, even if molecules do not have electroactivity properties.
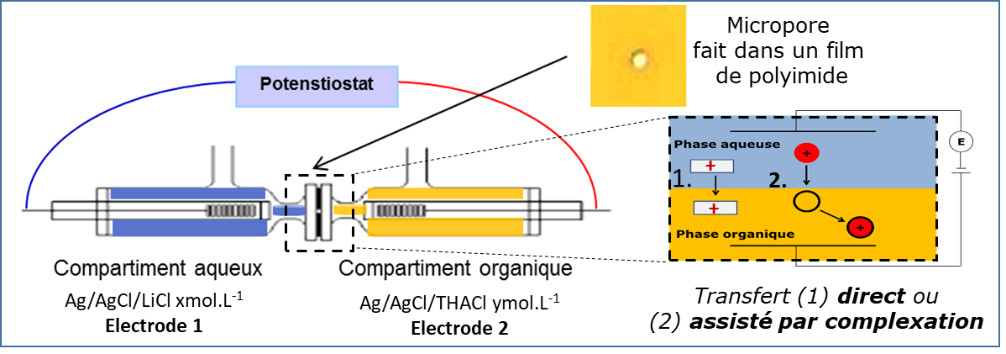
Schematic principle of measurements at liquid-liquid microinterfaces
Thus, organic compounds or alkaline earths can be detected and quantified by conventional electrochemical measurements. In addition, the major advantages of these devices are:
- no complex sample preparation
- measurements taken directly in waters of a very varied nature (little or not loaded or on the contrary very loaded with salts)
- no solid electrode (no clogging or need for regeneration)
- possibility to reach trace concentrations by using micropore networks
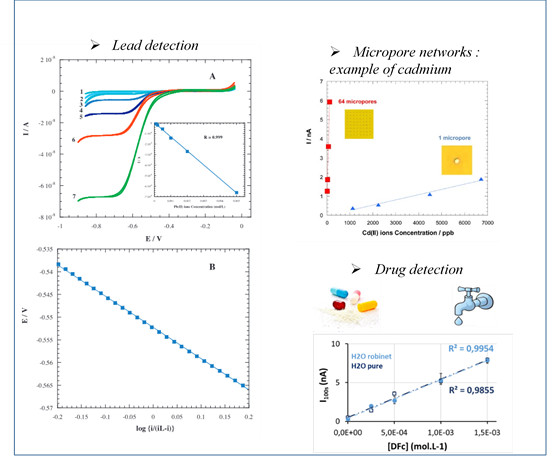
Examples of Measurements
These devices can be interesting both for targeted continuous flow applications, but also for acquiring fundamental data (liquid-liquid extraction, kinetics, nature and charge of the transferred species, etc.) essential for understanding the mechanisms of extraction and/or complexation.
Associated references :
- Publications
- S. Peulon, V. Guillou, et al. J. Electroanal. Chem. 2001, 514, 94-102.
- S. Peulon, Electrochim. Acta 2009, 54, 1537-1544.
- A. Mastouri, S. Peulon, D. Farcage, et al., Electrochim. Acta 2014, 120, 212-218.
- A. Mastouri, S. Peulon, et al., Electrochim. Acta 2014, 130, 818-825.
- C. Cannizzo, S. Peulon. Présentation orale et Article de Congrès, Journées Informations Eaux 2022, Apten , Poitiers (France)
- PhD Theses :
- A. Mastouri, 2014, Université d’Evry Val d’Essonne.
- Project
- Poc In Labs Paris-Saclay « Dispo » (2024)
NIMBE Research Axis





