At LCMCE, our research focuses on the circular carbon economy, using CO₂ and biomass as primary sources of carbon. More generally, our work focuses on the activation of oxygenated bonds, their use as platforms for the synthesis of high-value-added molecules, and understanding the reaction mechanisms involved.
Our approach is based on advanced catalytic methods (thermal, photochemical, or electrochemical) to interconvert C1 molecules, unlock the use of oxygenated molecules, develop renewable reducing agents, and valorize plastic waste.
Equipped with state-of-the-art analytical and synthesis platforms for molecular chemistry, our laboratory contributes to the Energy and Circular Economy research axis of the NIMBE.
C1 chemistry
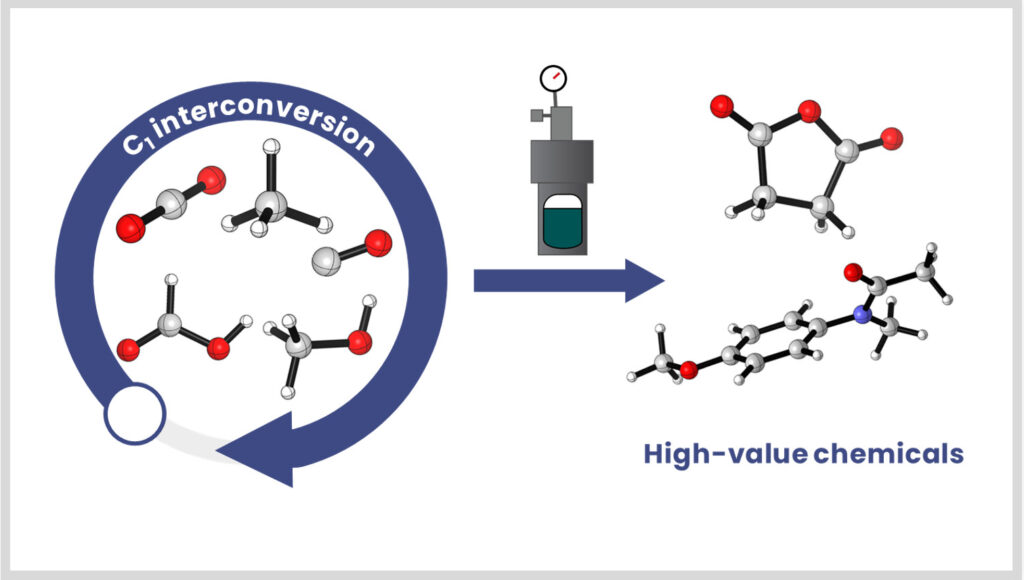
Our group develops integrated catalytic strategies to transform CO₂, even at low concentrations, and to interconvert C1 molecules (CO, HCOOH, formaldehyde). The thermocatalytic processes developed in-house are also coupled with other catalysis (electro-, photo-, etc.) through collaborations for the production of high-value-added molecules.
Deoxygenation
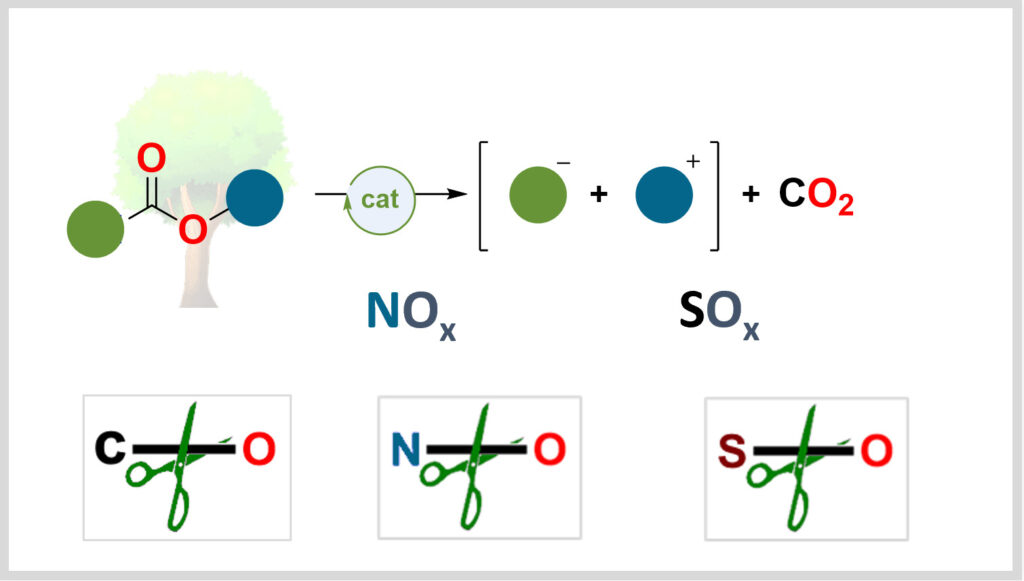
Activating oxygen bonds is central to the energy transition. Our laboratory develops catalytic methods to use these bonds as platforms for synthesizing high-value-added molecules.
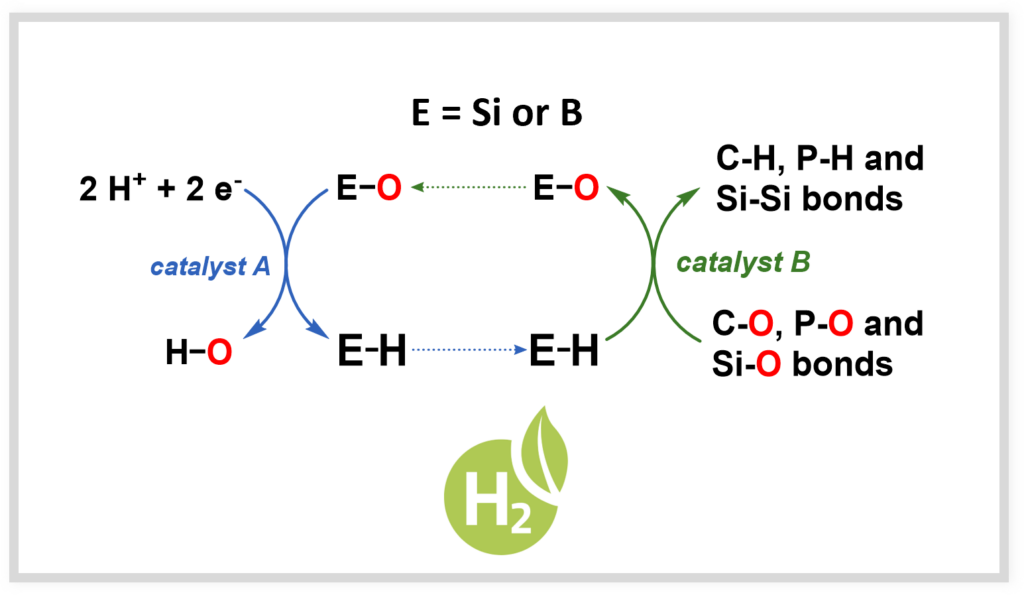
Renewable hydrides
To promote the circular economy, it is necessary to develop renewable reductants that can reduce oxygenated compounds in renewable carbon sources (e.g., CO₂, biomass). This work also paves the way for new developments in hydrogen storage.
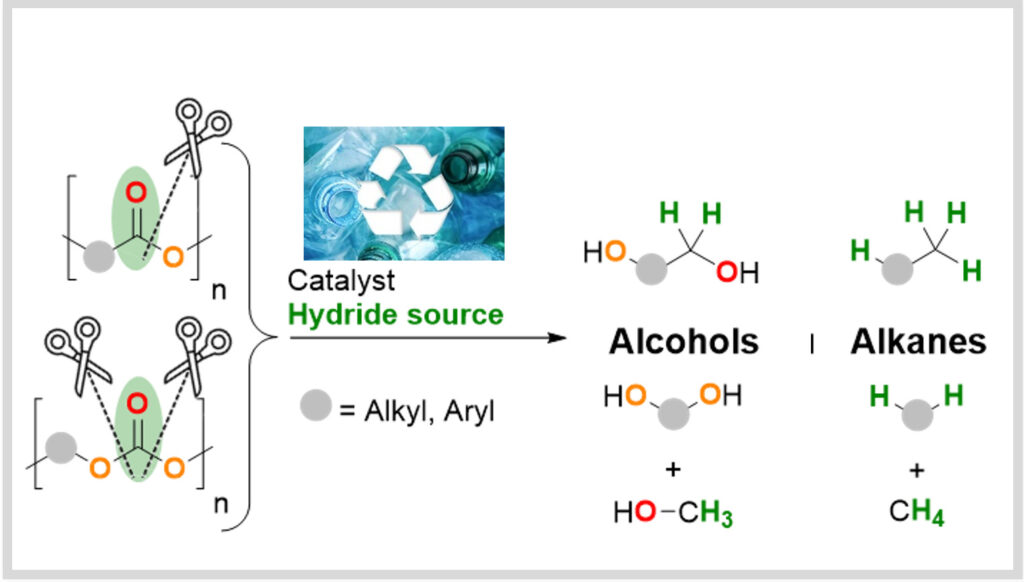
Depolymerization
The valorization of plastic waste (recycling, upcycling) as a renewable carbon source is also being studied at LCMCE. The team is currently developing strategies to leverage technologies developed in recent years for the upcycling of plastic waste.