J.-P Dognon, C Clavaguéra, and P. Pyykko
J.-P Dognon : CEA Saclay – IRAMIS/SCM (France)
C Clavaguéra : Laboratoire des Mécanismes Reactionnels, Ecole Polytechnique, CNRS, 91128 Palaiseau (France)
P. Pyykko : Department of Chemistry, University of Helsinki, Finland

Understanding the binding properties of a molecule is strongly linked to its number of valence electrons. The stability of metal complexes can be explained by an 18 electrons rule, widely used in the chemistry of transition metals. This rule stipulates that « the metal tends to accept, from the ligands that surround it, the number of electrons needed to close its valence layer by an optimal number of 18 electrons. The molecule thus acquires the electronic configuration of the noble gas that follows in the periodic table, namely (n-1) d10ns2np6. Is it possible to go beyond in the periodic table, i.e. to establish a similar rule for f elements (lanthanides and actinides) that could provide 14 additional electrons, thus making a 32 electrons rule?
The answer is yes! Theorists in chemistry within the CEA (Saclay), CNRS (Ecole Polytechnique, Palaiseau) and the University of Helsinki (Finland) had first proposed a system of 32 electrons: An @ Pb12 (An = Pu, Am+) through numerical simulation (quantum chemistry). This consists of a lead cage encapsulating an actinide. More recently, the same group of theorists has generalized this principle by offering a new family of compounds An @ C28. A quantum chemistry study has shown that such complexes could be stable and 32 electrons. Density functional theory has been used to study Th@C28, Pa+@C28, U2+@C28 et Pu4+@C28. These complexes have a tetrahedral symmetry with 16 « links » between the C28 cage and the central metal. Calculations show that the orbital 7s, 7p, 6d and 5f element of the central f hybridize with those of the cage to form a system with 32 electrons. The calculated thermodynamic properties show that the complex can form spontaneously in the gas phase. This was highlighted in the literature for U@C28, only compound experimentally characterized to date. Aggregates containing lanthanides, although stable, do not comply with the stated 32 electrons rule.
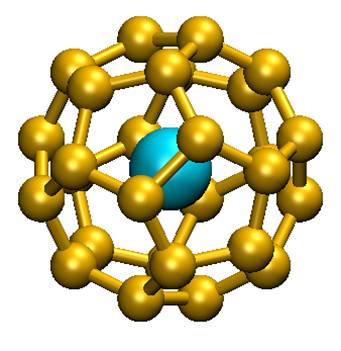
These f element-carbon aggregates are new compounds that can be used as building blocks for the assembly of nanomaterials with electronic, magnetic, optical or chemical well-controlled properties. The existence of this new class of endohedric atomic aggregates helped to transform a scientific curiosity (An@Pb12) in the statement of a new principle to 32 electrons.
This work has been the subject of two publications, one in Angew. Chem. Int. Ed. and another in J. Am. Chem. Soc.. The American Chemical Society has selected this work as the subject of a news in Chemical & Engineering News.
References :
| A predicted organometallic series following a 32-electron principle: An@C28 (An = Th, Pa+, U2+, Pu4+) J.-P Dognon, C Clavaguéra, and P. Pyykkö, J. Am. Chem. Soc. 131 (2009) 238. |
|
|
|
See also the news: « Stable Caged Actinides Proposed » by Jyllian N. Kemsley, Chemical & Engineering News, December 15, 86(50) (2008) 29. |
| Towards a 32-electron principle: Pu@Pb12 and related systems J.-P Dognon, C Clavaguéra, and P. Pyykkö, Angew. Chem.-Int. Edit. 46 (2007) 1427. |
15-01-2009




