Many complex molecular systems absorb light in the UV spectral range, including those of paramount biological importance, like DNA bases or proteins. The excited states created by UV absorption are endowed with mechanisms of deactivation which are of major importance for the photochemical stability of these species. The majority of these processes are ultrafast and provide a rapid and efficient way to dissipate the electronic energy into vibration, thus avoiding photochemical reactions.
In proteins, for instance, absorption in the near UV range is mainly due to the aromatic side chain of the tryptophan, tyrosine and phenylalanine amino acids and both spectroscopic and dynamic properties of these chromophores generally depend on their immediate environment, i.e., on the local conformation of the protein. In this context, synergetic theoretical/experimental studies of gas phase model peptides as proteins building blocks should lead to better understanding the photophysical phenomena involved in the relaxation mechanisms of proteins. Such an approach has been developed by a collaboration between the SBM team of LFP (CEA-CNRS URA2453), a theoretical team of the Ruđer Bošković Institut (Zagreb, Croatia) and two experimentalists of CLUPS (Paris Sud University, Orsay) in order to characterize the excited states of the stable conformers of a model peptide and establish the nonradiative relaxation mechanisms.1
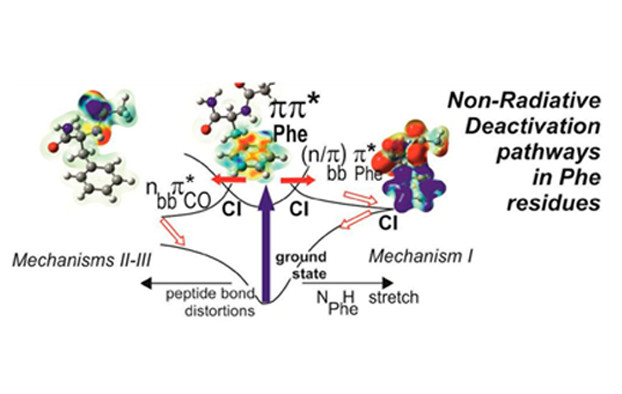
Sophisticated laser spectroscopic gas phase techniques developed recently in the SBM team and at CLUPS combining laser-desorption, supersonic expansion and IR-UV double resonance and UV-UV pump-probe techniques can provide conformation-selective data with a great deal of resolution; in particular the structure of each conformer thanks to their IR spectra, their transitions adiabatic energies, the lifetimes of their first excited state. In this spirit, dynamics relaxation of the first excited state of a capped peptide containing the phenylalanine amino acid, the N-acetylphenylalaninylamide (NAPA) which present three stable conformers (A, B and C) in the ground state2 have been investigated in the nanosecond regime at CEA and in the picosecond regime at CLUPS.
The measured lifetimes of the first excited state of the three conformers vary from 1 to 70 ns and depend strongly on the conformation of the peptide. In addition, the isotopic substitution of the hydrogen atoms of the NH and NH2 groups gives supplementary information on their involvement in the relaxation processes. Owing to the size of the system, their flexibility, the existence of non-covalent interactions which governs structures and the nature of the first excited states (locally excited as well as charge transfer states), the theoretical investigation of the relaxation mechanisms of the first excited states requires the use of sophisticated models.
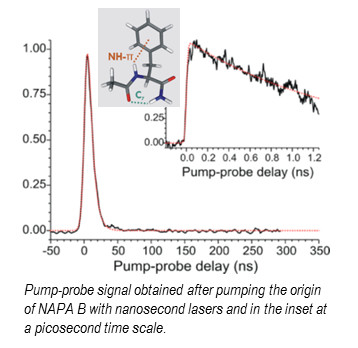
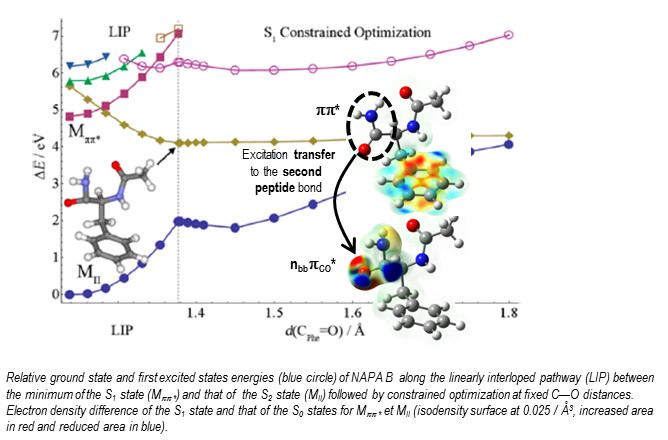
A double-step approach has been developed: (i) exploration of the potential energy surfaces of the first excited states of the conformers with a non-adiabatic dynamic using the time dependent density functional theory (NA-TDDFT) in order to identify the coordinates involved in the relaxation mechanisms and localize conical intersections, (ii) calculations of the energy profiles at a more refined level using the Coupled Cluster at second order (RI-CC2) and different strategies of optimization. The measured lifetime (1.5ns) of the first excited state pp* of the folded form (NAPA B), 50 times shorter than that of the extended form (NAPA A), are attributable to mechanisms involving an excitation transfer to a np* state induced by a distortion of the peptide bond.
Others mechanisms have been proposed to explain the very different behavior of the two other conformers (NAPA A and C). These first encouraging results thus open the way for a whole new field of research on the nonradiative deactivations which are ubiquitous in proteins. Synergetic theoretical/experimental studies are under progress in the SBM team in order to precise the impact of microsolvatation or the number of residues on these mechanisms. Conformer-selective measurements in the femtosecond regime are now targeted by the project DIRCOS gathering teams from SPAM and ISMO and funded by the LabEx PALM.
References :
1Unravelling the Mechanisms of Radiationless Deactivation in Model Peptides Following Photoexcitation of a Phenylalanine Residue. Malis, M., Ljubjic, I., Doslic, N., Loquais; Y., Gloaguen, E., Tardivel, B., Broquier, M., Jouvet, C., Brenner, V., Mons, M., 2013, J. Am. Chem. Soc, 134, 20340.
2Probing the competition between secondary structures and local preferences in gas phase isolated peptide backbones. W. Chin ; F. Piuzzi, I. Dimicoli and M. Mons, 2006, Phys. Chem. Chem. Phys. 8, 1033.
Contacts :


