Scientists at the “Laboratoire de chimie et biologie des métaux” (CEA-CNRS-Université J. Fourier, CEA-Grenoble), “Laboratoire de chimie des surfaces et interfaces” (CEA-Saclay) and a team at the Laboratoire d’innovation pour les technologies des énergies nouvelles et les nanomatériaux” (CEA Grenoble) have combined nanoscience and bio-inspired chemistry to develop, for the 1st time, a paltinum free material capable of catalyzing both production of hydrogen and its use in a fuel cell.
This result, important in view of a more competitive hydrogen economy is being published in the journal Science.
Among the new energy technologies, the use of hydrogen is an attractive solution. However, the hydrogen energetic chain cannot develop without the control of two key steps: first the production of hydrogen in large quantities by hydrolysis of water in devices called electrolyzers, and secondly the use of hydrogen in fuel cells to provide energy by its oxidation. Currently these processes require using platinum as a catalyst (substance that speeds up a chemical reaction). However, this metal is extremely rare (terrestrial abundance of about 5 ppm, equivalent to that of gold) and therefore very expensive. Getting rid of platinum and developing efficient catalysts that contains only abundant and cheap elements is a major challenge for the future of the energetic chain with hydrogen.
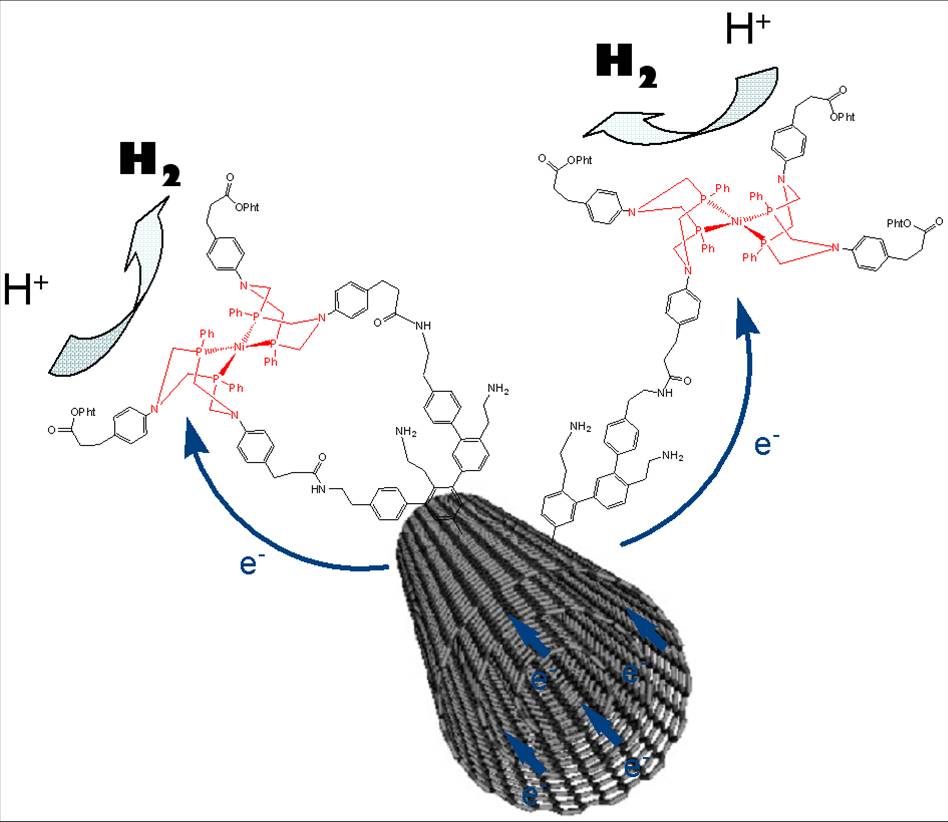
Researches for substituting platinum by abundant and low cost metals must look at the chemical processes at work in living organisms which, thanks to abundant metals like iron and nickel, know how to use hydrogen as an energy source, or to produce it from water. That approach may be called bio-inspired because to reproduce those natural processes, the researchers synthesized compounds based on nickel and iron, similar to the hydrogenase enzyme present in those systems.
However, to be used in technological devices, such catalysts would, like platinum, be set in very large quantities on electrodes. This requires wide available areas, which conventional materials do not offer. By their geometry that can significantly increase the potential surface for binding the catalyst, and by their high electrical conductivity, carbon nanotubes could be a solution to circumvent this difficulty.

In this study the researchers were able to bind one of these nickel-based bio-inspired catalysts, via its covalent grafting on carbon nanotubes, while improving the properties of this catalyst. The material so obtained has a promising catalytic activity for both the production and use of hydrogen. It turns out to be extremely stable and capable of operating in highly acidic environment which allows it to be compatible with the Nafion® membranes that are quasi-universally used in current hydrogen fuel cells. The development of this new material represents a new stage in the race for the improvement of the hydrogen solution for energy.
Reference:
From hydrogenase mimics to noble-metal free electrolytic nanomaterial for hydrogen evolution and uptake. A. Le Goff, V. Artero, B. Jousselme, P. Dinh Tran, N. Guillet, R. Métayé, A. Fihri, S. Palacin, M. Fontecave, Science, 326 (2009) 1384.
Communiqué de presse commun CEA – CNRS – Univ. J. Fourier (Grenoble).
Article in “Le Monde”Equipes de recherche :
- Laboratoire de Chimie et Biologie des Métaux, CEA – CNRS- Université Joseph Fourier – CEA Grenoble, 38054 Grenoble cedex 9.
- IRAMIS-SPCSI / Laboratoire de Chimie des Surfaces et Interfaces, CEA-Saclay, 91191 Gif sur Yvette Cedex
Contact:
Vincent Artero (DSV/IRTSV/LCBM) –
Serge Palacin et Bruno Jousselme (IRAMIS-SPCSI)