Elmar C. Fuchs1, Brigitte Bitschnau2, Jakob Woisetschläger3, Eugen Maier4, Brigitte Beuneu5 and José Teixeira5
1Wetsus, Center of Excellence for Sustainable Water Technology, Agora 1, 8900 CC Leeuwarden, The Netherlands
2Institute of Physical and Theoretical Chemistry, Graz University of Technology, Rechbauerstraße 12, 8010 Graz, Austria
3Institute of Thermal Turbomachinery and Machine Dynamics, Graz University of Technology, Inffeldgasse 25A, Graz, Austria
4Institute for Chemistry and Technology of Materials, Graz University of Technology, Stremayrgasse 16, 8010 Graz, Austria
5Laboratoire Léon Brillouin, CEA-CNRS/IRAMIS, CEA/Saclay, 91191 Gif-sur-Yvette Cedex, France.
In a highly surprising historical experience (1893), Sir William George Armstrong noted that it was possible to create a “water bridge” between two beakers connected by a cotton thread and exposed to a high voltage [1]. The bridge stood a few seconds after withdrawal of the wire.
Recently, a team of physicists from the University of Graz in Austria [2] showed that the bridge could be built spontaneously (and wireless!) between two beakers, applying a high voltage (~ 20 kV). This bridge may exceed one centimeter long with a diameter of few millimeters and a lifespan of about one hour.
This transient structure, caused by the intense electric field, is still very mysterious. Because of its chemical composition, the shape of its molecule and the inter-molecular hydrogen bond, the properties of water in liquid or solid phase are very specific. The nature of hydrogen bonds responsible for the stability of these structures is still under discussion, with significant consequences for our understanding of biology.
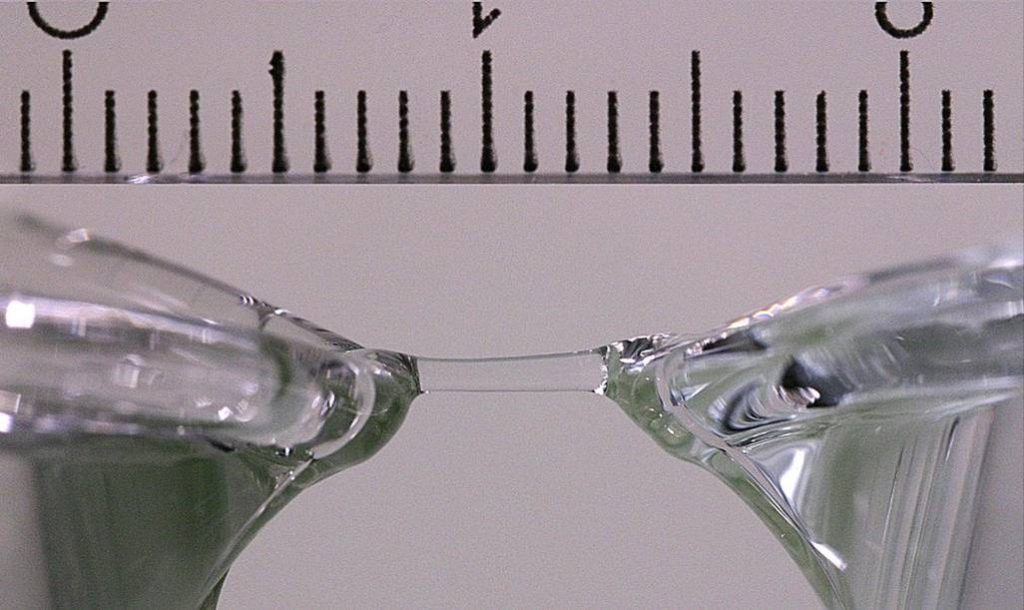
In an attempt to uncover the secret of these bridges, a first exploration of the molecular structure of the bridge by neutron scattering was performed at the Laboratoire Léon Brillouin (CEA / CNRS, Orphée reactor, 7C2 diffractometer for liquids and amorphous solids). Probing the matter with neutron scattering, gives information on local arrangement of atoms and molecules that constitute it. To improve the quality of the neutron signal, the use of heavy water (D2O) was mandatory. In a first step the team succeeded in achieving some “heavy water bridge”. The substitution of deuterium to hydrogen is known to let the structure of the liquid unchanged. One of the difficulties of the experience comes from the need for water of very high purity.
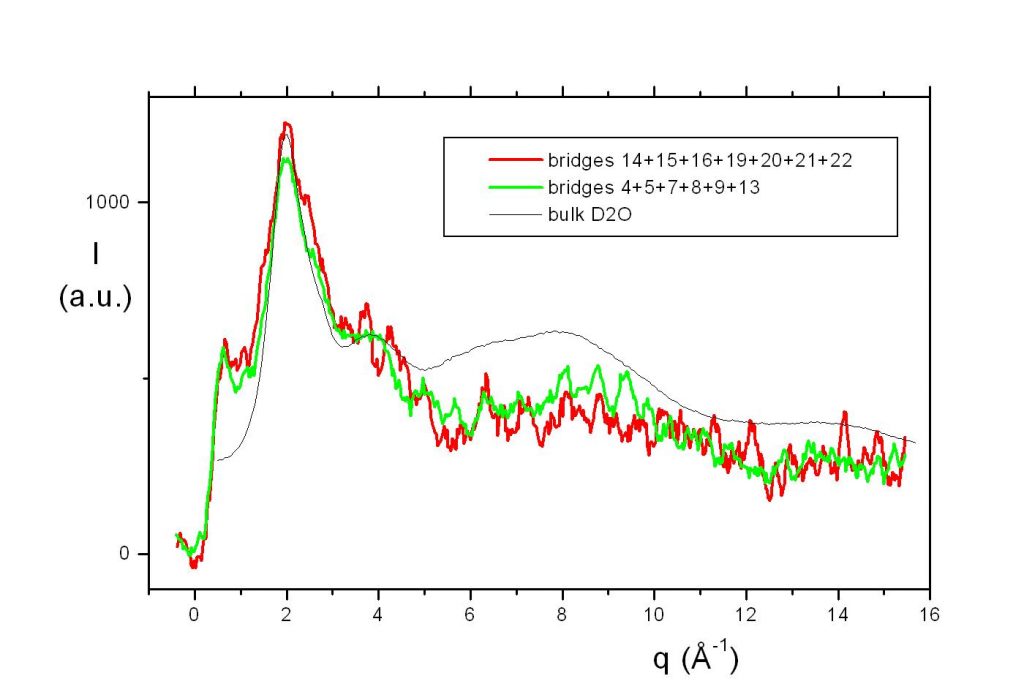
The coincidence of the main peak of the neutron scattering spectra for the bridge and for bulk D2O liquid water (see figure) means that there are no significant differences in the density and intermolecular distance of the molecules in the “bridge water”, compared to the normal structure of the liquid. This eliminates a number of hypotheses, such as, for example, those involving a collective orientation of the molecules. However, the scattering signal at small angles (<2 Å-1) differs significantly. This could be consistent with a structural order at intermediate distance or to the presence of heterogeneities in the liquid. This small angle scattering signal is also compatible with the presence of nano-bubbles. Their interfaces may play a role on the surface tension and on the stability of the bridge [4].
If a complete picture explaining the stability of the “water bridge” is still a mystery, it has been shown that it is possible to reproduce the experiment with heavy water and to probe its structure by neutron scattering, thus taking advantage of the high sensitivity of this technique to light elements (compared to that of X-rays). Further experiments of neutron scattering at smaller angles are planned and could provide crucial elements to solve the riddle.
References:
[1] W.G. Armstrong, Electrical phenomena The Newcastle Literary and Philosophical Society, The Electrical Engineer, 10 february 1893, p.154.
[2] E.C. Fuchs, J. Woisetschläger, K. Gatterer, E. Maier, R. Pecnik, G. Holler and H. Eisenkölbl, J. Phys. D: Appl. Phys. 40 (2007) 6112.
[3] E.C. Fuchs, B. Bitschnau, J. Woisetschläger, E. Maier, B. Beuneu and J. Teixeira, J. Phys. D: Appl. Phys. 42 (2009) 065502.
[4] E.C. Fuchs , K. Gatterer, G. Holler J. and Woisetschläger, J. Phys. D: Appl. Phys. 41 (2008) 185502.
This study was performed at LLB, with the high international prominence of the Laboratory in the study of the structure of water and its complex phase diagram and dynamics [5-7]. Beyond structural studies, one other important research topic concerns the hydration of proteins and other biological molecules.
[5] Physics of liquid water, structure and dynamics,
J. Teixeira and A. Luzar, Cours de l’Ecole de physique Les Houches
[6] L’étrange comportement de l’eau ultrafroide,
J. Teixeira, Pour la Science n°285, Juillet 2001.
[7] Dynamics of hydrogen bonds: how to probe their role in the unusual properties of liquid water,
J. Teixeira, A. Luzar and S. Longeville, J. Phys. Condens. Matter 18 (2006) S2353 (PDF).
|
|
Hydration Processes in Biology: Theoretical and Experimental Approaches, edited by M.C. Bellissent-Funel, NATO ASI Science Series A : Life Sciences Series (Publisher IOS Press), Vol 305 (Août 1999). |

