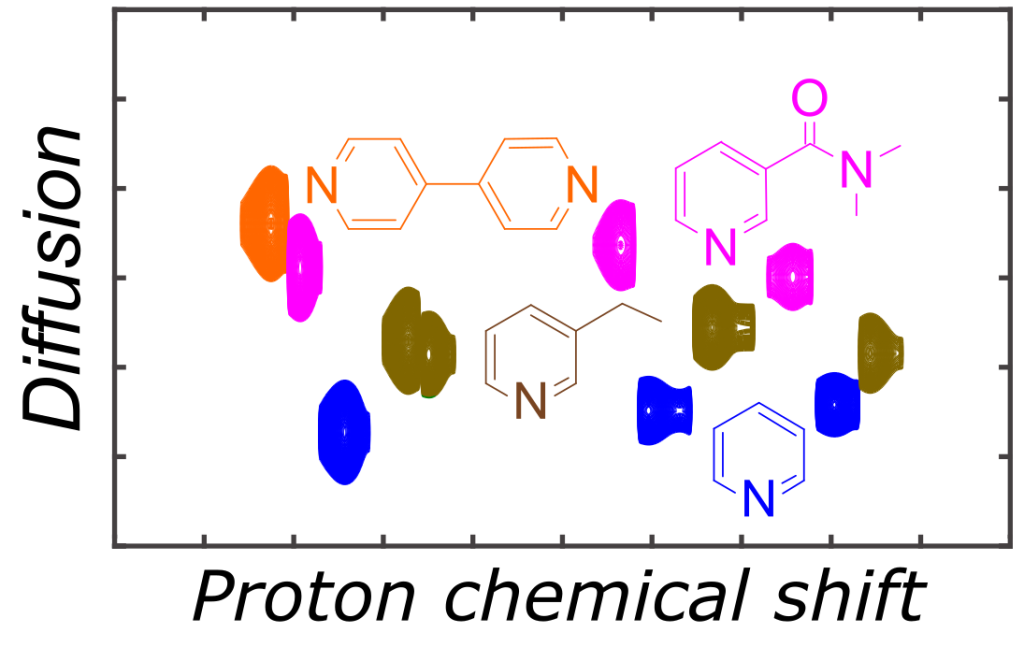
Hyperpolarization based on parahydrogen is one of the methods of choice to counteract the low sensitivity of NMR. It is based on the peculiar properties of this spin isomer of dihydrogen. Dihydrogen molecules exist as two sets of spin isomers, three states forming a triplet and called orthohydrogen, and one state called parahydrogen (Figure 1). When ortho and parahydrogen proportion are in a 3:1 proportion, a situation encountered at infinite temperature, the mixture cannot induce hyperpolarization.
Fortunately, one can take profit of two properties to produce and maintain an excess of parahydrogen.
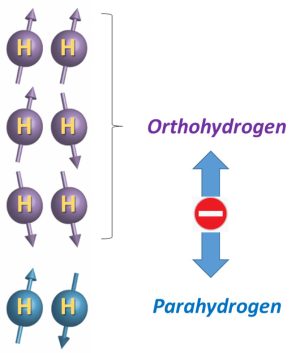
Figure 1 – The two spin isomers of hydrogen.
Firstly, by cooling dihydrogen at liquid nitrogen temperature over an appropriate catalyst, a 1:1 ortho and parahydrogen is reached. To further gain a factor 3 in resulting hyperpolarization, one can cool dihydrogen near 20 K resulting in almost 100% of parahydrogen. LSDRM owns two home-built set-ups for the enrichment in parahydrogen.
Secondly, for quantum reasons, ortho and parahydrogen molecules do not exchange each other unless the occurrence of a third molecule that acts as a catalyst. Room temperature storage of dihydrogen with significant excess of the para isomer in the gas phase for weeks has been demonstrated at room temperature.
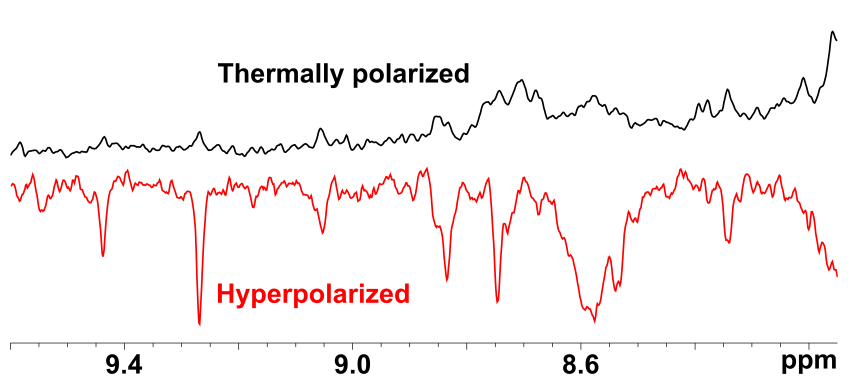
Pairwise addition of both hydrogens coming from parahydrogen on a metal or a multiple bond results in strong imbalance of population of nuclear spin states; in other words creates hyperpolarization. An example of proton NMR spectrum is given on Figure 2.
Hyperpolarization is short-lived, and its optimal use involves ultra-fast sequences based on spatio-temporal encoding. An example, where solutes are distinguished through their diffusion coefficient, is shown on Figure 3.