Au LCMCE, nos recherches s’inscrivent dans l’économie circulaire du carbone, en utilisant le CO₂ et la biomasse comme sources primaires de carbone. Plus généralement, l’activation des liaisons oxygénées, leur utilisation comme plateformes de synthèse de molécules à haute valeur ajoutée, ainsi que la compréhension des mécanismes réactionnels sont au cœur de nos travaux.
Notre approche repose sur des méthodes catalytiques avancées (thermiques, photochimiques ou électrochimiques) pour interconvertir les molécules C1, déverrouiller l’utilisation de molécules oxygénées, développer des réducteurs renouvelables, et valoriser les déchets plastiques.
Équipés de plateformes analytiques et de synthèse de pointe, nos travaux s’inscrivent dans les axes Énergie et Économie circulaire du laboratoire NIMBE.
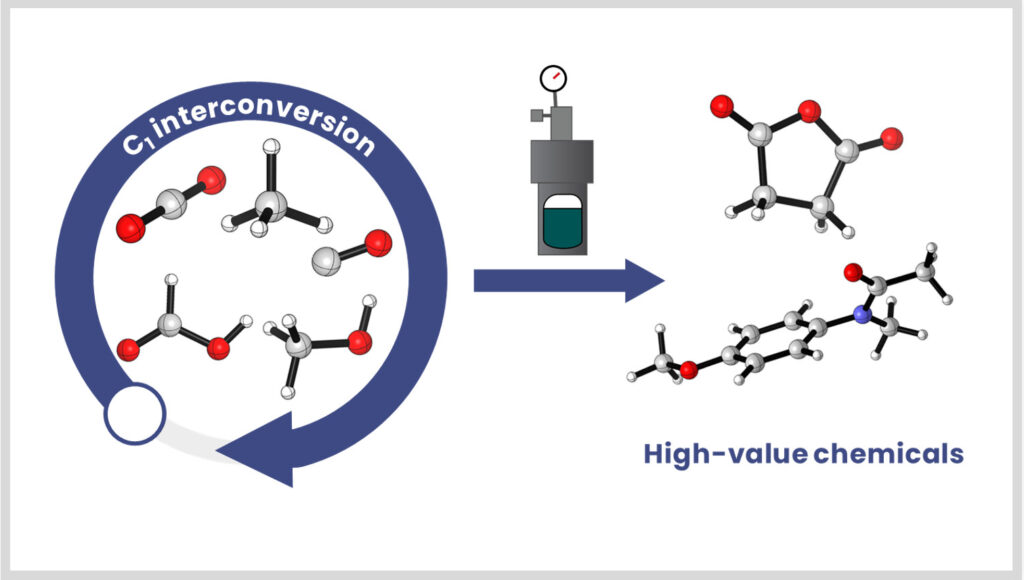
Chimie des molécules C1
Le LCMCE développe des stratégies catalytiques intégrées pour transformer le CO₂, même à faible concentration, ainsi que pour interconvertir les molécules C1 (CO, HCOOH, formaldéhyde). Les procédés thermocatalytiques développés en interne sont également couplés à d’autres catalyses (électro-, photo-, …) via des collaborations pour la production de molécules à haute valeur ajoutée.
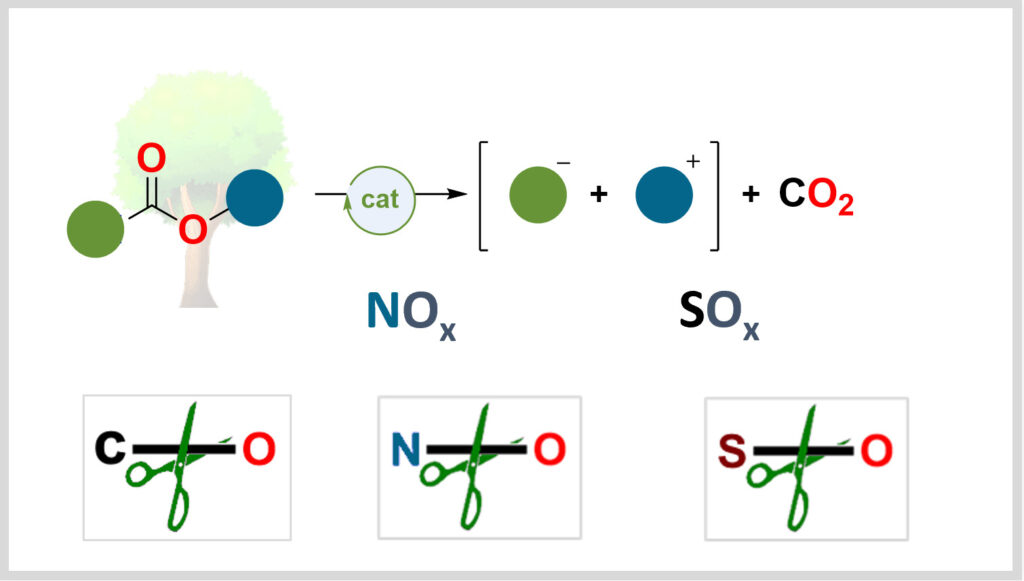
Désoxygenation
Activer les liaisons oxygénées est au cœur de la transition énergétique. Notre laboratoire développe des méthodes catalytiques afin d’utiliser ces liaisons comme plateformes de synthèse de molécules à haute valeur ajoutée.
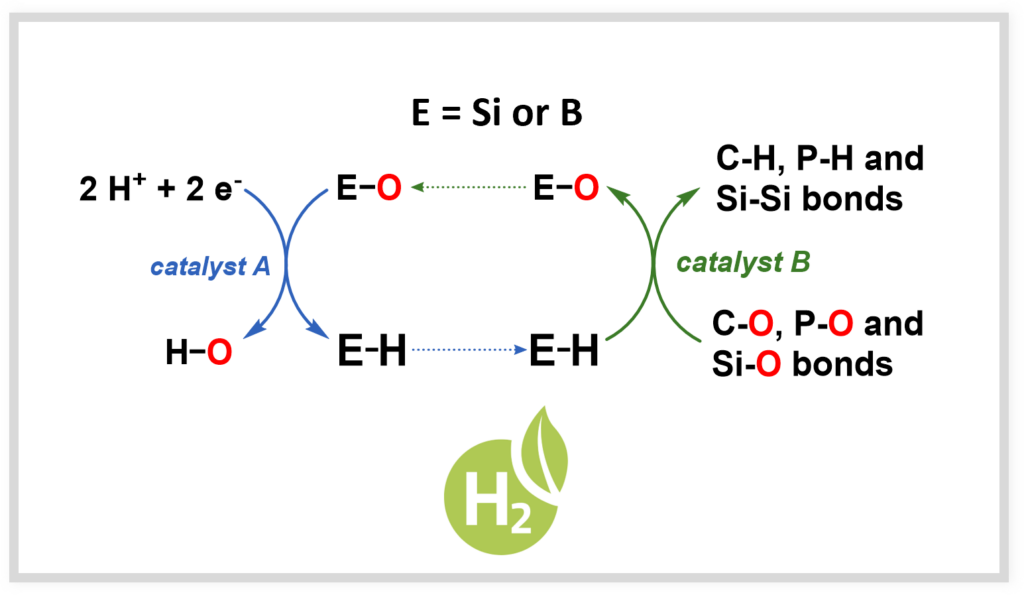
Hydrures renouvelables
Dans une optique d’économie circulaire, il est nécessaire de développer des réducteurs renouvelables, permettant de réduire les liaisons oxygénées présentes dans les sources renouvelables de carbone (CO2, biomasse…). Ces travaux ouvrent également la voie à de nouveaux développements sur le stockage de l’hydrogène.
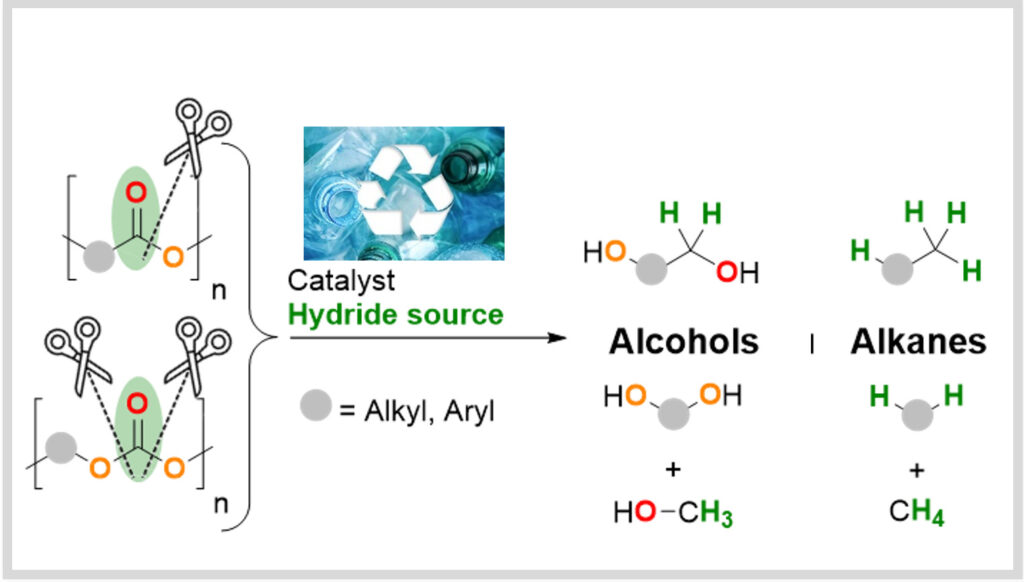
Dépolymerization
La valorisation des déchets plastiques comme source renouvelable de carbone est également étudiée au LCMCE. L’équipe s’attache actuellement à développer des stratégies de valorisation des technologies développées ces dernières années pour le surcyclage de déchets plastiques.





