Simeon Minić, Burkhard Annighöfer, Arnaud Hélary, Laïla Sago, David Cornu, Annie Brûlet, Sophie Combet, Biophysical Journal 121(13) (2022) 2514.
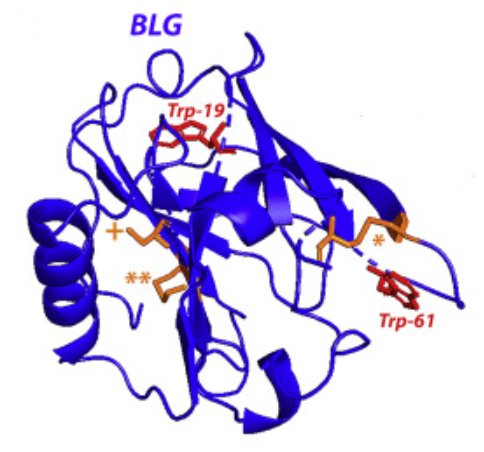
High pressure (HP) is a particularly powerful tool to study protein folding/unfolding, revealing subtle structural rearrangements. Bovine β-lactoglobulin (BLG), a protein of interest in food science, exhibits a strong propensity to bind various bioactive molecules. We probed the effects of the binding of biliverdin (BV), a tetrapyrrole linear chromophore, on the stability of BLG under pressure, by combining in situ HP small-angle neutron scattering (SANS) and HP-UV absorption spectroscopy. Although BV induces a slight destabilization of BLG during HP-induced unfolding, a ligand excess strongly prevents BLG oligomerization. Moreover, at SANS resolution, an excess of BV induces the complete recovery of the protein “native” 3D structure after HP removal, despite the presence of the BV covalently bound adduct. Mass spectrometry highlights the crucial role of cysteine residues in the competitive and protective effects of BV during pressure denaturation of BLG through SH/S-S exchange.
A presentation in French on Scoop.it! of the Paris-Saclay University.