Salah Bouazizi, Salah Nasr & Marie-Claire Bellissent-Funel
MD simulation and analysis of the pair correlation functions, self-diffusion coefficients and orientational correlation times in aqueous KCl solutions at different temperatures and concentrations,
S. Bouazizi, S. Nasr and M.-C. Bellissent-Funel, J Solution Chem (2024) https://doi.org/10.1007/s10953-024-01366-8
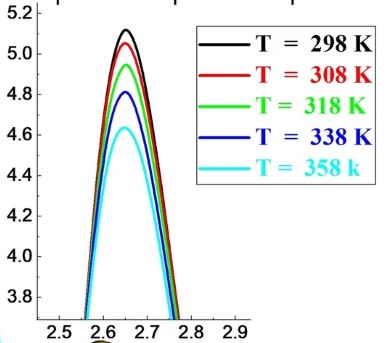
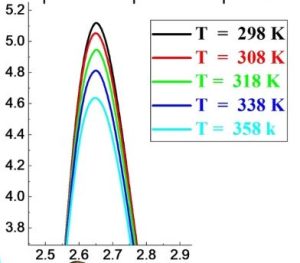
Abstract : In this study, we investigate some structural and dynamical properties of aqueous KCl solutions at different temperatures and concentrations. We study a 1.6 mol·kg–1 aqueous KCl solution at five temperatures and five concentrations at ambient conditions only. Molecular dynamics simulations with the flexible SPC water model were conducted to characterize all partial pair correlation functions, the velocities auto-correlation ones, and the dielectric constants. The analysis of the water pair correlation functions shows a disruption of the H-bond network and a decrease of the oxygen-hydrogen coordination number as temperature or salt concentration increases. The increase of each parameter favors the exchange of molecules between the first and the second hydration shells. Ions pair correlation functions show principally that the fraction of K+-Cl− contact ion pairs increases and that of separated ion pairs decreases with increasing temperature or concentration. For all particles, the values of the calculated self-diffusion coefficients rise with temperature and fall with salt concentration. The self-diffusion coefficients of K+ and Cl− tend to towards each other at high concentration. Temperature or salt concentration causes a drop in the dielectric constant. For all studied temperatures or salt concentrations, the calculated ratio of the orientational correlation times τ1/τ2 for the OH vector indicates that the motion of water molecules can be accounted for by an angular jumps model.