Plastic waste released into the environment breaks down into micro- and nano-particles. A growing number of exploratory missions and studies show that these particles are now present in many environmental compartments. Now invisible to the naked eye, they represent a hidden facet of plastic pollution, whose influence on living organisms is still poorly understood.
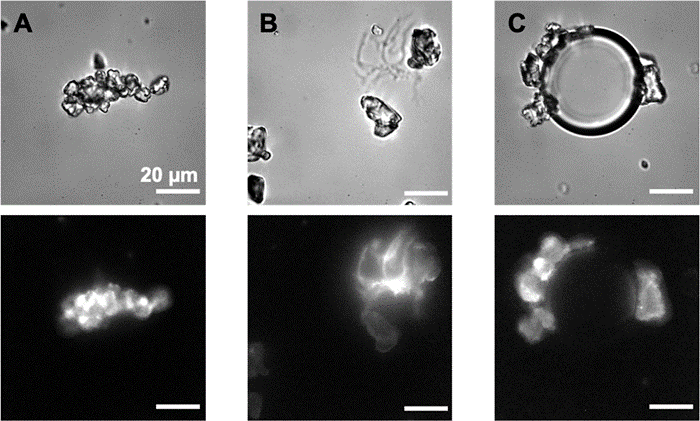
Plastic fragments are naturally hydrophobic, and the study carried out by two teams from NIMBE and I2BC at CEA, in association with INRAE and IMMM, shows that the addition of a crown of biological molecules enables them to be dispersed in water
It was then shown that, while this layer favors the entry and dispersion of microplastics in the environment, it also makes it possible to propose “low tech” strategies for collecting them.
Micro- and nanoplastics (MNP) are an emerging form of diffuse pollution that is causing a great deal of environmental concern. In the absence of knowledge of their physical and chemical properties and their biological interactions, it is still difficult to fully assess the extent and impact of this pollution.
Teams from I2BC and NIMBE at CEA, in association with IMMM, Université Paris Cité (BFA ) and Synchrotron Soleil, have therefore combined their skills to study in depth the behavior of model microplastics in water, and the role that proteins adsorbed to their surface may play (formation of a “corona”)
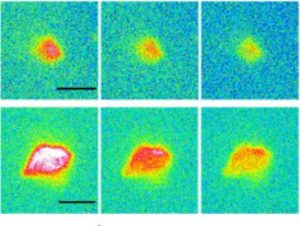
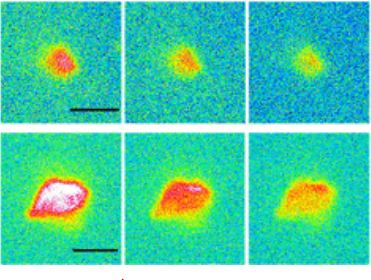
The studies focused more specifically on the behavior of microparticles (5 and 10 µm in diameter) of polypropylene and polyethylene, the most persistent plastics in the environment, in the presence of proteins. Firstly, it was observed that without proteins, buoyancy and capillary forces tend to gather the particles towards the surface (“creaming”), and very few particles remain in solution. In contrast, biomolecules act as a surfactant, rendering plastics hydrophilic, allowing them to diffuse in water and favoring their dispersion. In addition, the corona generates a globally repulsive interaction between particles, preventing them from agglomerating. After 24 hours without agitation, the quantity of microplastics suspended in water can remain above1011 particles per liter. The stabilization observed increases with the structural quality of the protein corona, which was assessed by imaging on the Disco line of the Soleil synchrotron.
Image of polyethylene microplastics aggregated by proteins:
Top, optical microscopy images. Below, images of the proteins present in the same aggregates, obtained by far-UV fluorescence microscopy at the Synchrotron Soleil.
In addition to simply observing the behaviour of plastic particles in the presence of proteins, this study shows that by controlling the protein corona, it is possible to manipulate microplastics in solution. Two ways of destabilizing the protein corona were tested:
- precipitation, induced by high salt levels (ammonium sulfate, saturated solution, 5.3 M (NH4)2SO4),
- heat treatment (~80°c / 1h) leading to protein aggregation.
Under these 2 conditions, the corona of the microparticles is strongly destabilized, resulting in the creaming of around 95% of the microparticles after 24 hours.
This easy-to-use, low-tech thermal treatment could be of interest, for example, for purifying water and making it drinkable, where industrial depollution solutions are not available. It should also be noted that this method is similar in principle to traditional methods of wine clarification by “fining”, where egg white proteins (albumin) coagulate on contact with tannins, extracting undesirable particles initially in suspension.
Would the same strategy also work for nano-sized particles (nanoplastics), which are more complex to study? Their larger surface area should favour protein adsorption, and therefore the possibility of controlling their stability in solution by direct action on the protein corona. What remains to be done is to conduct the experiments, using other methods better suited to particles of this submicron size…
References
M. Schvartz, F. Saudrais, S. Devineau, J-C Aude, S. Chédin, C. Henry, A. Millán-Oropeza, T. Perrault, L. Pieri, S. Pin, Y. Boulard, G. Brotons & J-P Renault. Scientific Reports volume 13, Article number: 1227 (2023).
See CEA Joliot-IRAMIS highlight:“Instability of proteins in the presence of plastics“.
________________________________________
- CEA-IRAMIS contact: Jean-Philippe Renault (NIMBE/LIONS)
- CEA-JOLIOT contact: Yves Boulard(I2BC-S/SB2SM/LBSR)
Collaboration :
- M. Schvartz, F. Saudrais, O. Taché, J. Leroy, S. Chédin, L. Pieri, S. Pin, J-P Renault, Université Paris-Saclay, UMR NIMBE CEA-CNRS, France
- F. Saudrais , , K. Rakotozandriny, S. Chédin, Y. Boulard, Université Paris-Saclay, UMR I2BC CEA-CNRS.
- M. Schvartz, G. Brotons, Institut des Molécules et Matériaux du Mans (IMMM), UMR 6283 CNRS, Le Mans Université.
- S. Devineau, Functional and Adaptive Biology Unit (BFA – UMR 8251 CNRS – Université Paris Cité).
- Frédéric Jamme, Synchrotron Soleil.