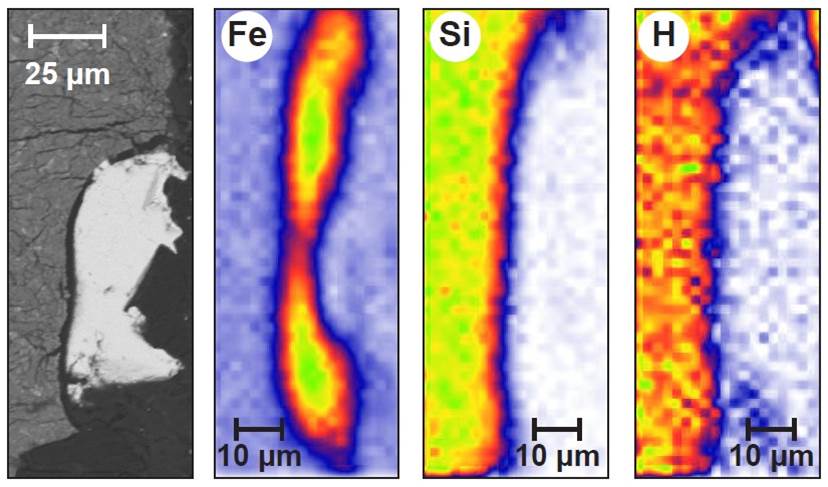
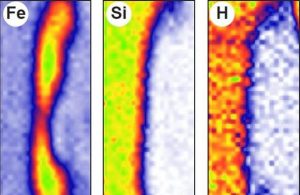
How much hydrogen is hidden in the core of telluric planets (such as Earth or Mars)? To answer this difficult question, a collaboration involving the LEEL team at UMR NIMBE has simulated the laboratory segregation of an iron-rich alloy in a silicate environment, recreating pressure and temperature conditions analogous to those of the formation of the Earth’s core. Mapping of the various elements using the NIMBE nuclear microprobe shows that only a tiny quantity of hydrogen must have incorporated the core of telluric planets, promoting the early formation of a water-rich mantle and atmosphere.
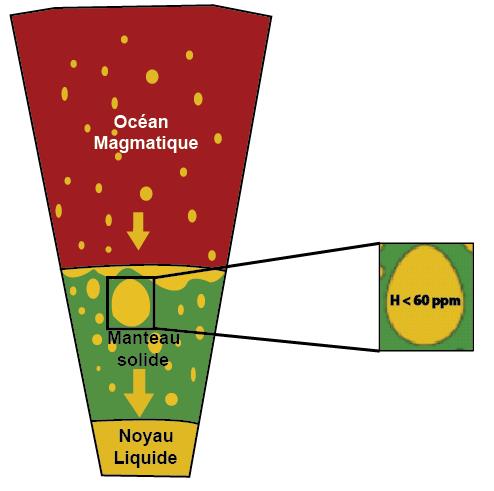
Some telluric planets, such as Earth and Mars, have a metallic core at their center, composed essentially of iron and surrounded by a rocky (silicate) mantle. Such a core was formed in the planet’s first millions of years, when it was still so hot that its surface was melted over a thickness of several hundred kilometers, forming a magmatic “ocean”. In this ocean, iron, the heaviest of the abundant elements, sinks deep down to form the core. As it migrates towards the center of the planet, it reacts with the magma and combines with light elements such as oxygen, silicon and sulfur. The result is a central core of liquid iron, agitated by movements, from which a planetary magnetic field can emerge.
But what exactly is the chemical composition of the Earth’s core? This is one of the major challenges that scientists have been trying to tackle for several decades. Hydrogen, for example, is said to have accompanied the segregation of iron during core formation and to have become an important element of the core. But is this really the case?
In an attempt to solve this problem, researchers have simulated the formation of a “mini-nucleus” in the laboratory, recreating conditions analogous to those prevailing during the formation of an authentic planetary nucleus: pressure of 5 – 20 GPa and temperature of 1,700 – 2,500°C. The separation of the iron-rich metal alloy (core) from the silicate (mantle) in the presence of water (1.5% by weight) is monitored on the “multianvil” press at the Magmas Volcanoes Laboratory (LMV, Puy-de-Dôme). Once the experiment is complete, the composition of light elements, in particular hydrogen, must be measured in each silicate and metal sample. For this purpose, ERDA (Elastic Recoil Detection Analysis) ion scattering, as available on the UMR NIMBE* nuclear microprobe, proved to be the only method that allowed this analysis, and more specifically the local determination of hydrogen in absolute terms and with the necessary precision.
The result: hydrogen is one of the elements least likely to enter the nucleus, even at very high pressure. It is prevented from doing so by its preferential association with other light elements such as silicon, carbon and sulfur. It is only found in the metallic part at a low level of the order of a few hundred ppm (parts per million).
Based on the high estimates obtained from these experiments, scientists conclude that the concentration of hydrogen in the core is less than 70 ppm (parts per million). Most of the available hydrogen therefore remains associated with the constituents of the mantle, from where it can be released at the surface to form an atmosphere rich in dihydrogen and hence water, by reaction with the oxygen also present in abundance.
References
Low hydrogen contents in the core of terrestrial planets,
V. Clesi, M.A. Bouhifd, N. Bolfan-Casanova, G. Manthilake, F. Schiavi, C. Raepsaet, H. Bureau, H. Khodja & D. Andrault, Sciences Advances 4 (2018) e1701876.
* The nuclear microprobe of UMR NIMBE’s LEEL team is accessible by experiment request, possible at any time during the year.
CEA contact: Hicham Khodja, IRAMIS/NIMBE – Laboratoire d’Etude des Eléments Légers (LEEL)
Collaboration:
- Laboratoire Magmas et Volcans (LMV) CNRS, Universités Blaise Pascal et Jean Monnet, Observatoire de physique du globe de Clermont-Ferrand, Institut de recherche pour le développement,
- Laboratoire d’Étude d’Éléments Légers UMR 3685 NIMBE CEA-CNRS, Université Paris-Saclay,
- Institut de Minéralogie, de Physique des Matériaux et Cosmochimie, UMR 7590 CNRS-IRD-IMHN-UPMC, Université Paris-Sorbonne.