Unravelling a mechanism of action for a cecropin a‑melittin hybrid antimicrobial peptide: the induced formation of multilamellar lipid stacks
T. Silva, B. Claro, B. F. B. Silva, N. Vale, P. Gomes, M.-S. Gomes, S. S. Funari, J. Teixeira, D. Uhríková, and M. Bastos, Langmuir, 2018, 34 (5), pp 2158–2170.
An understanding of the mechanism of action of antimicrobial peptides is fundamental to the development of new and more active antibiotics. In the present work, we use a wide range of techniques (SANS, SAXD, DSC, ITC, CD, and confocal and electron microscopy) in order to fully characterize the interaction of a cecropin A-melittin hybrid antimicrobial peptide, CA(1-7)M(2-9), of known antimicrobial activity, with a bacterial model membrane of POPE/POPG in an effort to unravel its mechanism of action.
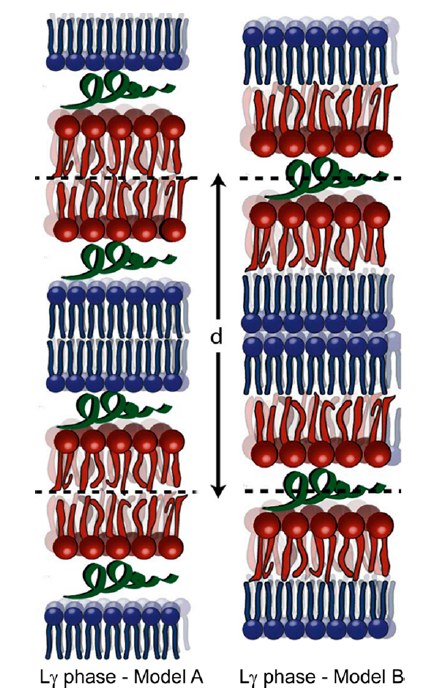
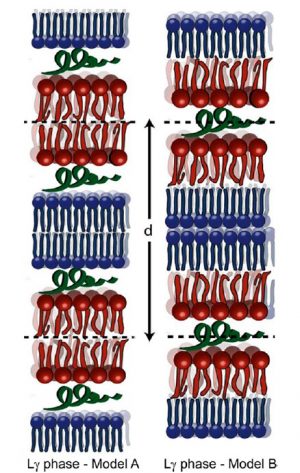
We found that CA(1-7)M(2-9) disrupts the vesicles, inducing membrane condensation and forming an onionlike structure of multilamellar stacks, held together by the intercalated peptides. SANS and SAXD revealed changes induced by the peptide in the lipid bilayer thickness and the bilayer stiffening in a tightly packed liquid-crystalline lamellar phase. The analysis of the observed abrupt changes in the repeat distance upon the phase transition to the gel state suggests the formation of an Lγ phase. To the extent of our knowledge, this is the first time that the Lγ phase is identified as part of the mechanism of action of antimicrobial peptides. The energetics of interaction depends on temperature, and ITC results indicate that CA(1-7)M(2-9) interacts with the outer leaflet. This further supports the idea of a surface interaction that leads to membrane condensation and not to pore formation.
As a result, we propose that this peptide exerts its antimicrobial action against bacteria through extensive membrane disruption that leads to cell death.
http://dx.doi.org/10.1021/acs.langmuir.7b03639