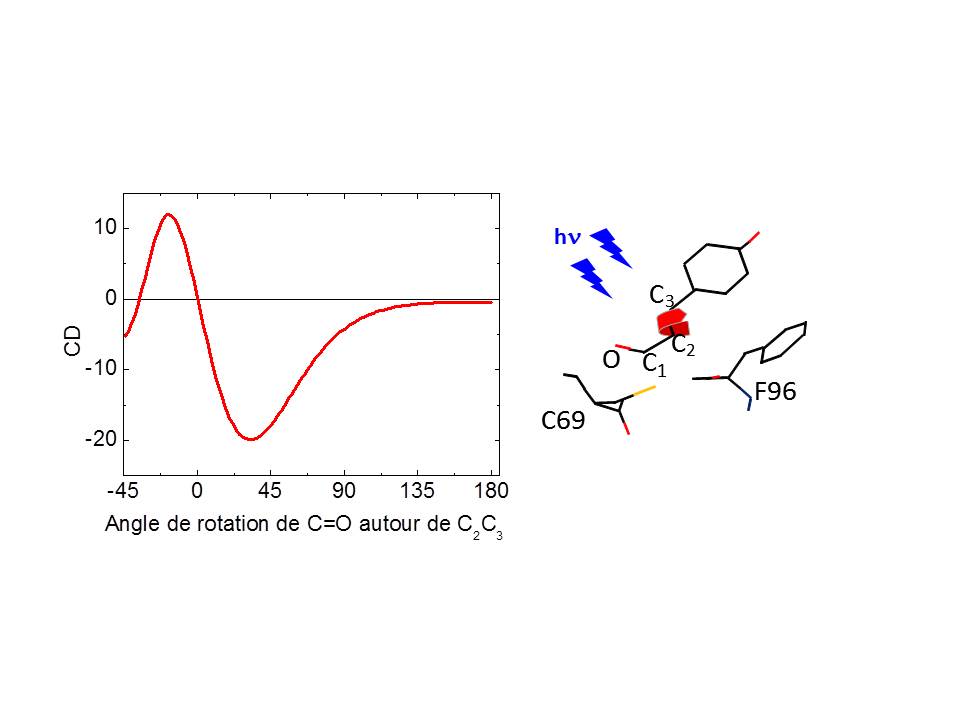
Motions of the carbonyl group of the Photoactive Yellow Protein (PYP) chromophore have been investigated, for the first time, by femtosecond time-resolved circular dichroism (tr-CD) spectroscopy, in the near-ultraviolet spectral region. The quantitative analysis of the tr-CD signals shows that, upon excitation, the carbonyl group undergoes a fast (≪0.8 ps) and unidirectional flipping motion in the excited state with an angle of ca. 17−53°. For the subset of proteins that do not enter the photocycle, tr-CD spectroscopy provides strong evidence that the carbonyl group moves back to its initial position, leading to the formation of a nonreactive ground-state intermediate of trans conformation. The initial ground state is then restored within ca. 3 ps.
CEA Contact : Pascale CHANGENET-BARRET
Lucille Mendonça, François Hache, Pascale Changenet-Barret, Pascal Plaza, Haik Chosrowjan, Seiji Taniguchi, and Yasushi Imamoto
J. Am. Chem. Soc., 2013, 135 (39), pp 14637–14643

