V. Geertsen
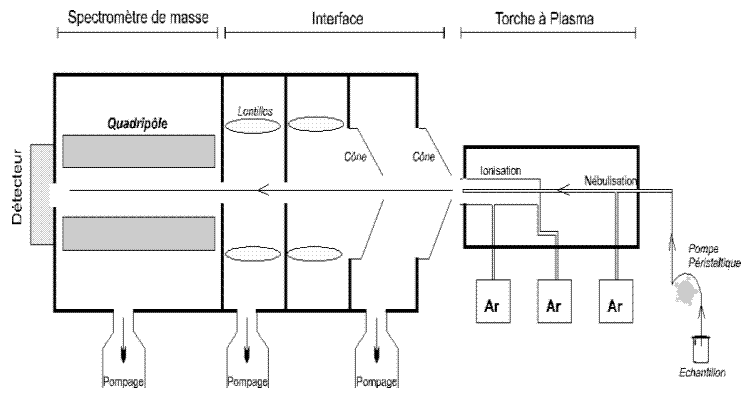
The mass spectrometry is an instrumental technique of analysis resting on separation, the identification and the quantification of the components of a sample according to their mass. It is founded on the coupling of a plasma generating ions and of a quadrupolar mass spectrometer (in the case of the ICP-MS Thermoelectron X7) which separates these ions in mass.
The ICP-MS analysis of the samples can be divided into four stages: introduction-atomizing, ionization, separation in mass, detection. The sample is put in solution. An autosampler (standard HAVE 90) coupled to a peristaltic pump introduces the solution into a vaporization chamber where the nebuliser transforms it into a liquid aerosol made up of micro-droplets of some µm using argon. Thus the aerosol formed is sent in an argon plasma torch (15 l.min—1) at very high temperature (between 6.000 and 10.000 °C), sufficient to vaporize, dissociate, atomize and ionize completely the majority of the elements. Part of this plasma (10%) is sampled by a first opening of 1 mm diameter approximately at the top of a platinum or nickel cone (“the sampler”), then scatter under the effect of the moderate vacuum (1-2 mbar) in a pumping chamber (which makes it possible to pass from the atmospheric pressure to the secondary vacuum of the mass spectrometer) and passes then in a second opening (“the skimmer”). A system of differential vacuum accelerates the ions of plasma towards a whole of electrostatic lenses which extracts the positively charged ions and transports them towards a quadrupolar filter of mass. This whole of lenses is also called ionic lens.
The detection part is carried out thanks to a electrons multiplier with discrete dynodes. For the detection of the positive ions, a series of dynodes is subjected to a negative tension of a few thousands of volts. The end of the series of dynodes is connected to the ground. Against the exit of the quadrupole, a positive ion, attracted by the negative tension, runs up against the semiconductor surface of the first dynode. This positive ion causes the emission of one or several secondary electrons which again run up against the wall of the second dynode: a “snowball” effect occurs. At the end of the series of dynodes, for one ion which runs up against the detector, approximately 100 electrons reach a collector equipped with a preamplifier. The signal is translated into pulse repetition frequency (counts), a data-processing interface ensures the transfer of the data so that they are treated. For a given isotope, the number of ions measured makes it possible to directly calculate the concentration of the element analyzed thanks to quantitative and qualitative software. The counts are converted into concentrations with two types of calibrations: external (standard solutions) and intern (spikes). For the complex matrix such as rocks, an additional data processing is necessary.
The ICP-MS became essential for the simultaneous analysis of the elements in traces and “ultra-traces” (elements lower than 10—6g/g) and for the determination of the isotopic ratios in rocks, water, sediments, biologic samples. In “routine”, it is possible to analyze, in a few minutes, 20 to 30 elements in the most varied materials. In addition, it has an excellent sensitivity, enabling to detect elements present at the level of (1 ppt is a part = 10—12g/g) in a solution of rock or in water. Without any chemical separation, it allows the analysis of many elements in trace the level of ppb (10—9g/g). Precision varies from element to another according to the potential of ionization and the studied matrix, the average uncertainty “of routine” being lower than 3%.