Understanding the formation of nanoparticles in water at room temperature is a major challenge, both to tackle the mechanisms of mineral growth in biological systems, and to synthesise efficiently nanomaterials. However, the growth mechanisms usually invoked sometimes fail dramatically to describe reality, especially the final size, the crystalline quality and rate of production of the nanocrystals. Progress in this area are slow because of the great difficulty in observing the intermediate steps between the solution before reaction, and the construction of the first nanocrystal. A group of researchers from the IRAMIS/NIMBE, the Laboratory of Condensed Matter Physics at the École Polytechnique and the Université Pierre-et-Marie-Curie has managed this feat by using in particular the SOLEIL synchrotron radiation. Their observations allowed to propose a formation scenario which is unusual, but consistent with reality, and probably very generic. These results have been recently published in ACS Nano.
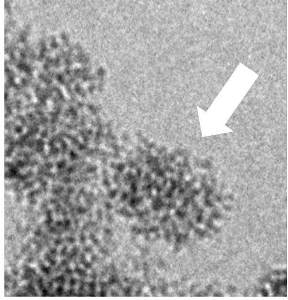
The synthesis in water and at room temperature of crystalline oxide nanoparticles involves many transient states between the ions in solution and the nanocrystals. For these syntheses, the classical theory of nucleation can not fulfill its usual role, as a guide to the control of the final size, since it ignores the intermediate steps before the construction of the first nucleus. More modern theories must therefore take into account these transient states. But the knowledge on these intermediate states is scarce because of the great difficulty to observe in situ the relevant sizes and time scales (e.g. d = 1-50 nm and t < 100 ms). In this context, a group of researchers from the IRAMIS/NIMBE, the Laboratory of Condensed Matter Physics at the École Polytechnique, and the Université Pierre et Marie Curie has been able to characterise in situ the formation of luminescent nanoparticles made of yttrium vanadate doped with europium (YVO4:Eu) from aqueous precursor solutions (salts of yttrium/europium nitrate and vanadate). The combination of synchrotron radiation (SWING beamine in SOLEIL), rapid mixing tools (millifluidic) and electron microscopy after quenching in liquid ethane (cryoTEM) has been used to characterize the transition from the ionic solution to the aqueous suspension of nanocrystals
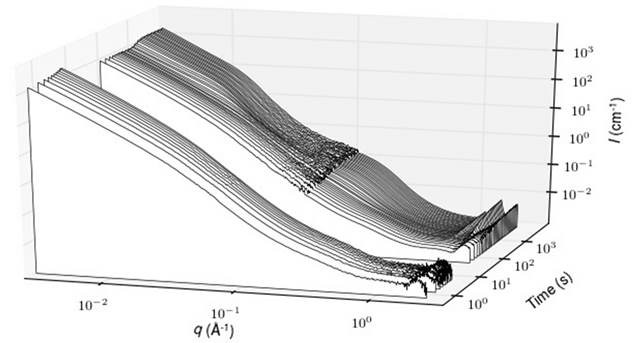
This shows that the formation of these YVO4:Eu nanoparticles takes place in two steps: first, a nanostructured amorphous phase precipitates (t < 400 ms), which then crystallizes. The major finding of this study is that the transient amorphous phase is already nanostructured (aggregates of 30 nm composed of nanometer-scaled grains), and that this nanostructure constraints the shape of the final nanoparticles (40 nm aggregates, consisting of nanoscale crystalline grains). There is therefore a “template” effect, i.e. an initial constraint which imposes the final shape. This result is significant as (i) many synthetic nanocrystals have the same morphology, suggesting the very generic nature of our observations, and (ii) the template effect is by contrast absent from observations conducted on biologic minerals (calcium phosphate), since in that case the grains of the amorphous phase coalescence prior to crystallisation.
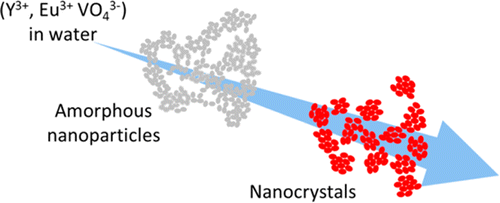
The observations made on these luminescent nanoparticles first reinforce the modern theories of nucleation, then allow identification of effective levers to guide the nanostructure of a broad class of oxides synthesized in aqueous phase, either inspired by the observations performed on these synthetic YVO4:Eu nanoparticles, or on nanocrystals produced in biological systems.
Référence :
Amorphous to crystal conversion as a mechanism governing the structure of luminescent YVO4:Eu nanoparticles
Blaise Fleury†‡, Marie-Alexandra Neouze†‡, Jean-Michel Guigner§, Nicolas Menguy§, Olivier Spalla‡, Thierry Gacoin†, and David Carriere‡*
ACS Nano, 2014, 8 (3), 2602
Contacts for CEA: Blaise Fleury, Olivier Spalla, David Carriere.



