Most polymers are today still made from depleting fossil fuels which, combined with their environmental persistence, make them unsustainable. In this talk (in French!), I will describe the use of sugars as a renewable resource for the creation of novel polymers.
We have developed a procedure, for the synthesis of 6-membered ring cyclic carbonates from 1,3-diols and CO2 as precursors, under mild conditions (room temperature, low CO2 pressure), which presents a viable alternative to the traditional use of phosgene derivatives for monomer synthesis.[1] We have applied this synthetic route to the various sugar derivatives (e.g. from mannose,[2] thymidine[3] and 2-deoxyribose[4]), resulting in several novel cyclic carbonate monomers. CO2 can even be used to invert the natural configuration of sugars and yield monomers not possible by other methods. Subsequent controlled Ring-Opening Polymerisation (ROP) of these new monomers using organocatalysts or metal precursors yields the corresponding polymers (aliphatic polycarbonates, APCs) bearing a sugar ring structure in their backbone, and presenting competitive thermal properties. ROP proceeds at room temperature in solution or in the melt under more industrially relevant conditions. Copolymerisation and functionalisation can be used to further tune the polymer properties. CS2 can also be used instead of CO2 to produce sulfur-containing polymers (e.g. poly(xanthates)), with alternative properties [5].
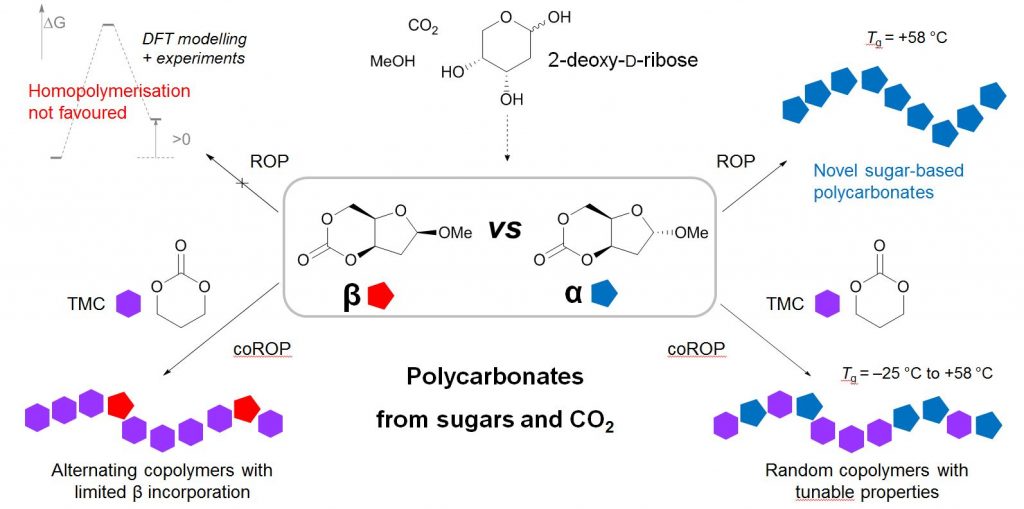
Figure 1. Example of polycarbonates made by ring-opening (co)polymerisation of cyclic carbonates derived from CO2 and 2-deoy-D-ribose.
Finally, I will talk about our most recent efforts to combine fatty acids and sugar feedstock towards new elastic polymers, as well as to branch out of ROP and of homogeneous catalysis altogether.
[1] G. L. Gregory, M. Ulmann and A. Buchard, RSC Adv., 2015, 5, 39404–39408; T. M. McGuire, E. M. López-Vidal, G. L. Gregory and A. Buchard, J. CO2 Util., 2018, 27, 283–288.
[2] G. L. Gregory, L. M. Jenisch, B. Charles, G. Kociok-Köhn and A. Buchard,
Macromolecules, 2016, 49, 7165–7169.
[3] G. L. Gregory, E. M. Hierons, G. Kociok-Köhn, R. Sharma and A. Buchard,
Polym. Chem., 2017, 8, 1714–1721.
[4] G. L. Gregory, G. Kociok-Köhn and A. Buchard, Polym. Chem., 2017, 8, 2093–2104.
[5] E. M. López-Vidal, G. L. Gregory, G. Kociok-Köhn and A. Buchard, Polym. Chem., 2018, 9, 1577–1582.
University of Bath, Department of Chemistry, Centre for Sustainable Chemical Technologies