V. Padmanabhan, J. Daillant, L. Belloni, S. Mora, M. Alba, and O. Konovalov
The study of aqueous salt solutions continues to attract various research groups because of their fundamental importance in various physicochemical, biological and atmospheric processes. The air/water interface plays a crucial role in such processes and differs to a large extent when compared to bulk. To further understand the role of the interface, direct access to the surface excess or the knowledge of the concentration profiles of ions will not only improve our present understanding but also help to predict the properties associated with it. Ions, though of the same valency, tend to interact differently with proteins (salting in or salting out as predicted by Hofmeister) or differ in their degree of adsorption at the air-water interface. In recent times, there have been considerable efforts by various research groups using different sophisticated surface sensitive probes to understand the organisation of the ions and its impact on the solvent features and also through molecular dynamic simulation.
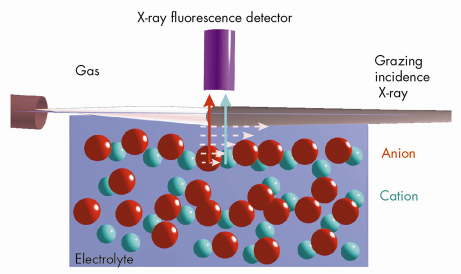
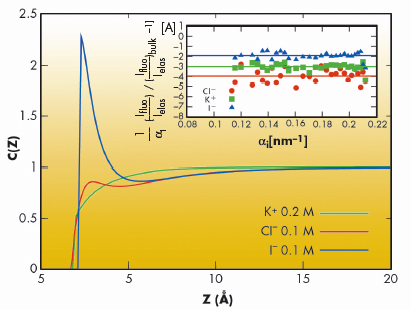
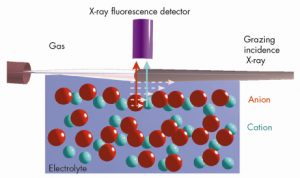
We have employed grazing incidence X-ray fluorescence to investigate an electrolyte solution under ambient conditions (Figure 49) at beamline ID10B. The X-ray fluorescence from the ions (chemical sensitive) is registered as a function of the angle of incidence (depth sensitive). Systematic investigations on acids, salts and their mixtures at different bulk concentrations yield quantitatively their surface excess. Theoretical analysis using a hyper-netted chain approximation to predict the concentration profile (Figure 50) invoke the necessity to include the short range surface-ion interaction potential in addition to the dispersion and the long range electrostatic interactions. Furthermore, the competition in ion adsorption for a mixture of 0.1 M KCl and 0.1 M KI reveal clearly the enhancement of iodide relative to chloride anion (Inset of Figure 50). We could apply this approach of analysis to 20 different experiments using 4 fit parameters. The strength of the effective potential deduced from such analysis were, -4.0 kBT, -4.7 kBT, -4.4 kBT and -3.15 kBT, for Cl–, Br–, I– and H+ (at contact), respectively. Our studies highlight the importance of the short range interactions at the interface of aqueous electrolyte solutions.
Principal publication and authors
V. Padmanabhan (a), J. Daillant (a), L. Belloni (a), S. Mora (b), M. Alba (a), and O. Konovalov (c), Phys. Rev. Lett. 99, 086105 (2007).
(a) CEA Saclay, Gif-sur-Yvette (France)
(b) UMR 5587 CNRS, Université Montpellier II (France)
(c) ESRF