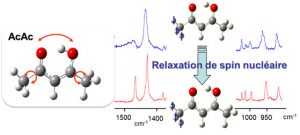
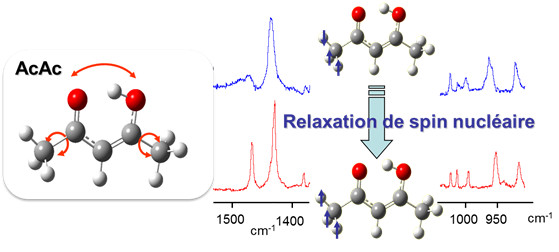
The stability and conformational dynamics of biomolecules are strongly influenced by the dynamics of hydrogen bonds and by hydrogen transfer processes which occur through these bonds. Not surprisingly, a large activity develops since many years to understand which molecular deformations play a role when a hydrogen atom is transferred through a hydrogen bond. The acetylacetone molecule (noted AcAc in the figure) is often used to approach this question from a fundamental point of view. Under the form drawn in the figure (the most stable one in the gas phase), AcAc is indeed one of the simplest molecule which carries an intramolecular hydrogen bond with the H-atom in proper position to transfer from an atom to another (here, the two oxygen atoms drawn in red in the figure). This issue relates to a more general one in reaction dynamics, a field in physical chemistry: to bring enough an accurate experimental material for modeling and foreseeing how several degrees of freedom couple together to drive to a chemical reaction.
The MOMA group of the Institute of the Molecular Sciences of Orsay (ISMO), in collaboration with the Reaction Dynamics group of the laboratory Francis Perrin (LFP) developed a very attractive method to show (maybe to control in the future) how large amplitude motions are coupled to frustrated rotations of the methyl groups in AcAc (schemed as bend arrows in the left panel of the figure) to stimulate the transfer of the H-atom between the two oxygen atoms (also shown by an arrow in the figure). This collaboration was initiated by the ANR project GOUTTELIUM and is pursued through the NOSTADYNE project funded by Triangle de la Physique. The AcAc molecule was isolated in a matrix of para-hydrogen, a quantum solid that does not perturb the deformation of hosted molecules. At the temperature of the matrix (4K), the relaxation of the nuclear spin of the methyl groups is very slow. This has been taken as an advantage to reveal that the pseudo-rotation of the methyl groups is intricate with the large amplitude motion associated with the H-atom transfer. The experimental method that lead to this result (see the reference) appeared as an elegant way, although indirect, to answer a long controversy on the role of the methyl rotations in the H-atom transfer in AcAc. The issue was important actually, since AcAc is considered as a prototype to unravel the dynamics of molecules possessing an intramolecular hydrogen bond.
Reference
Nuclear Spin Conversion to Probe the Methyl Rotation Effect on Hydrogen-Bond and Vibrational Dynamics Rolando R. Lozada-Garcia, Justinas Ceponkus, Michèle Chevalier, Wutharath Chin, Jean-Michel Mestdagh, Claudine Crépin*
Angewandte Chemie (http://dx.doi.org/10.1002/anie.201200727) 51, 6947–6950 (2012)
Contacts
Claudine Crépin :
Institut des Sciences Moléculaires d’Orsay – UMR 8214
Bâtiment 210
Université Paris-Sud
91405 Orsay Cedex
Tel : 33 (0)1 69 15 75 39 ou 82 67
Fax : 33 (0)1 69 15 67 77
Jean-Michel Mestdagh
Laboratoire Francis Perrin
Service des Photons Atomes et Molécules – Bat 522
Tel :2-25 45
Fax : 2-87 07