The description of the interactions controlling the shape of a protein is crucial in understanding the cellular mechanisms, but is still difficult to achieve on biological systems because of their complexity.
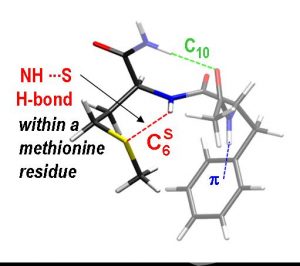
In this context, the use of model molecules makes accessible to experiments many biological problems lying at the heart of current societal issues. Gas-phase IR/UV spectroscopy of small peptides is an outstanding example. The study of peptides containing the methionine residue has recently shown that NHamide—Smethionine hydrogen bonds are particularly strong. They are, for example, as strong as the “classical” NHamide—OCamide hydrogen bonds which define the secondary structure of proteins.
Analysis of protein structures identified to date reveals that the type of NHamide—Smethionine bond observed experimentally occurs on 12% of methionines. Comparison of the parameters defining the NHamide—Smethionine bond shows that the hydrogen bonds formed in the gas phase faithfully reproduce those observed in proteins. This strongly suggests that the properties of the NHamide—Smethionine bonds highlighted in the gas phase, especially their strength, are identical to those naturally occurring in proteins.
The study of the constraints imposed to the backbone by these NHamide—Smethionine bonds shows that they reduce the range of possible values for the Ramachandran angles. This phenomenon common to gas-phase peptides and proteins having these NHamide—Smethionine bonds thus illustrates the effect that these bonds can have on the rigidity of the peptide backbone. These results already open interesting paths in understanding the way antitumor drugs act. Journal of Physical Chemistry Letters 2012, 3, 755−759
Michel MONS – 01 69 08 20 01
Eric GLOAGUEN – 01 69 08 35 82