Dans les systèmes biologiques, l’intérieur des cellules est un milieu très encombré, qui peut provenir de la présence de macromolécules inertes ou non vis-à-vis des réactions biologiques (encombrement macromoléculaire) ou de la séquestration physique par des éléments tels que des réseaux de fibres et de membranes (confinement). Par rapport au in vitro, l’encombrement in vivo peut considérablement affecter le comportement des protéines et des acides nucléiques (conformation, stabilité, cinétique de repliement…).
L’équipe biophysique du LLB/MMB s’intéresse à l’influence de l’encombrement de macromolécules sur :
- les conformations des protéines (influence de la pression, de polymère hydrophobes), la stabilité, la réduction de la mobilité et l’implication sur des facteurs physiologiques connexes, la transition bobine-hélice des chaînes polypeptidiques,
- le repliement et la compaction des acides nucléiques,
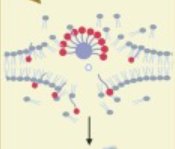
- les interactions entre les membranes cellulaires et diverses molécules (peptides, protéines telles que la dystrophine, ions, gaz), interactions qui sont à l’origine de nombreux processus biologiques (fonction musculaire, réparation des cassures double-brin de l’ADN).
Un modèle bactérien est particulièrement étudié, la protéine Hfq qui affecte la dynamique du génome, en ayant des implications directes sur l’efficacité de la machinerie cellulaire (réplication, transcription, traduction). Sont notamment étudiés les interactions de membranes de phospholipides avec des nanopores, des protéines amyloïdes et des complexes contenant des fragments d’AND et des cations divalents (Lipoplexes) pouvant agir comme agent de thérapie génique. La diffraction de neutrons donne également accès à la structure cristallographique des protéines, en particulier lorsqu’elles sont deutérées.
Ces diverses études sont abordées via diverses méthodes de biochimie, de biologie moléculaire et de biophysique, dont les méthodes de diffusion statiques (DXPA et DNPA), de diffusion inélastiques des neutrons (diffusion quasi-élastique incohérente en utilisant la substitution isotopique, scans élastiques), de dynamique moléculaire, de dichroïsme circulaire (conventionnel ou sur synchrotron), de spectroscopie infrarouge, de rhéologie et l’imagerie (fluorescence, microscopie électronique ou microscopie de force atomique). L’étude des membranes est également abordée en surface par réflectivité de neutrons et de rayons X.