
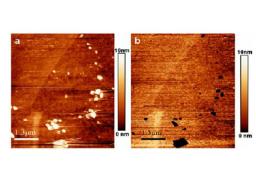
Fig. 1 : Contact mode AFM micrographs of an austenitic stainless steel (304L) at the free corrosion potential (a) and at the pitting
corrosion potential (b). Pits (arrows) in fig 1b are growing on the location of protrusion observed in fig1a. The nature of these bumps
remains unknown but it could be oxide/hydroxide compounds. (Size 5μmx5μm)
The early stages of corrosion of stainless steels under stress are still not very well understood despite a lot of investigations. [1] Many techniques have been used for studying this process of metal damaging because of its huge economical importance. However, none of these investigations, except optical microscopy, implies in situ observations and provides maps of damaged areas. Thus, the degradation if these metals was studied with a poor resolution, typically above the micrometre. As a consequence, the onset of corrosion is believed to appear randomly along the metal surface. The uprising of local probe microscopy in the 90’s and specially the atomic force microscopy (AFM) has opened new avenues for in situ exploration of these damaging processes at sub micrometre scale.
In this work, damages induced by corrosion were investigated in situ by using a new and original setup, which combines an AFM microscope working in contact mode and an electrochemical cell. We will present surface modifications due to corrosion without an external stress applied to the sample.
Two kinds of stainless steels were studied: an austenitic steel (304L) and a duplex one containing an austenite and a ferrite phases (Uranus 50). As we want to detect defects in the nanometre range, the specimen preparation presents many stages including mechanical and electrochemical polishing with a final mechano-chemical polishing. This last treatment has been performed with colloidal silica particles for about 20 h. From AFM micrographs 5μm x 5μm in size, the best results characterizing the surface roughness are ~ RMS 0.5nm.
The first stages of corrosion of stainless steels covered by a passive layer have been studied by in situ AFM in chloride containing solutions at room temperature. The solution contains 0.5M of NaCl and 0.01M of borate salts. The specimen was immersed in solution and a - 400 mV potential (vs.Ag/AgCl) was applied during 300s. Then the potential was raised at a low rate (1mV s-1) until pitting occurred as shown by the intensity increase in the electrochemical cell. Both 304L and Uranus 50 stainless steels exhibit a free corrosion potential at -230 ± 25mV (vs. Ag/AgCl) in these operating conditions.
We first explore the relief changes induced by the formation of pits. Fig.1 presents a typical morphology of the 304L steel surface before and after pitting. At +230mV, pits are visible on the surface. They are randomly dispersed along the surface of grains while some of them are located on grain boundaries. One notices that these pits appear exactly where protrusions were observed at the free corrosion potential. Such a correlation means that some local modifications of the passive film favour the pit formation. [2, 3]
The pit distribution along the 304L steel surface contrasts with the one observed in the duplex stainless steel. On this metal, a very low density of pits is detected and the pits appear at the boundary between austenite and ferrite grains. On 304L, we evidenced the influence of local strained areas on the corrosion process. On a specimen exhibiting an initially flat surface prepared without electrochemical polishing, we notice that the majority of pits are located along lines. This arrangement finds its origin in the presence of strained rays in the surface layer which favour the pit formation. As shown in [2], surface areas associated with strained rays present a higher sensitivity to corrosion than the other parts (without local strained areas) of the well polished surface. As a consequence, the pits initiate along these rays and grow, in some cases, by coalescing in elongated pits.
One of the main advantages of AFM investigation of the corrosion process is the possibility to study in situ the progress of pits. Although AFM tip cannot probe the bottom of pits, this technique provides information on the kinetics of the evolution of pit depth at the pitting potential. Fig. 2 illustrates the progress of different pits in 304L steel in contact with a 0.5M NaCl solution at a potential of 0.25V (vs. Ag/AgCl). During these observations, the pits become deeper with small changes in their XY dimensions. Considering the high resolution in Z of AFM, the rate of corrosion was measured within a range smaller than 100nm. We found a rate of progression equal to 0.019nm s-1. As the overall shape of the pits is known, the density of current can be evaluate and compare to values obtained from theoretical calculations of corrosion models [2, 4].
REFERENCES :
[1] P. Lacombe et al., Les aciers inoxydables, Les Editions de Physique (1990)
[2] F. A. Martin, Ph.D Thesis Paris VI (2005)
[3] F. Martin et al., Passivation of Metals and Semiconductors, and Properties of Thin Oxide Layers, Edts P. Marcus and
V. Maurice, Elsevier Science, (June 2006)
[4] F. Martin, et al., Local probe techniques for corrosion research h: (EFC 45), Edts R. Oltra, V. Maurice, R. Akid and
P. Marcus, Woodhead Publishing Limited, Cambridge (ISBN: 1 84569 236 5 [ISBN-13: 978 1 84569 236 0], February
2007)
•  Les archives de l'IRAMIS et du DRECAM / Archives of DRECAM and IRAMIS › Matériaux, surfaces et nanostructures
Les archives de l'IRAMIS et du DRECAM / Archives of DRECAM and IRAMIS › Matériaux, surfaces et nanostructures
• Laboratory of Physics and Chemistry of Surfaces and Interfaces • Service de Physique et Chimie des Surfaces et des Interfaces
• Laboratory of Oxide Surfaces and Interfaces • Laboratoire des Interfaces et Surfaces d'oxydes (LISO)
