Sophie Combet, Françoise Bonneté, Stéphanie Finet, Alexandre Pozza, Christelle Saade, Anne Martel, Alexandros Koutsioubas, Jean-Jacques Lacapère, Biochimie 205, (2023) 61-72
The translocator protein (TSPO) is a ubiquitous transmembrane protein of great pharmacological interest thanks to its high affinity to many drug ligands. The only high-resolution 3D-structure known for mammalian TSPO was obtained by NMR for the mouse mTSPO in DPC detergent only in presence of the high-affinity PK 11195 ligand. An atomic structure of free-ligand mTSPO is still missing to better understand the interaction of ligands with mTSPO and their effects on the protein conformation.

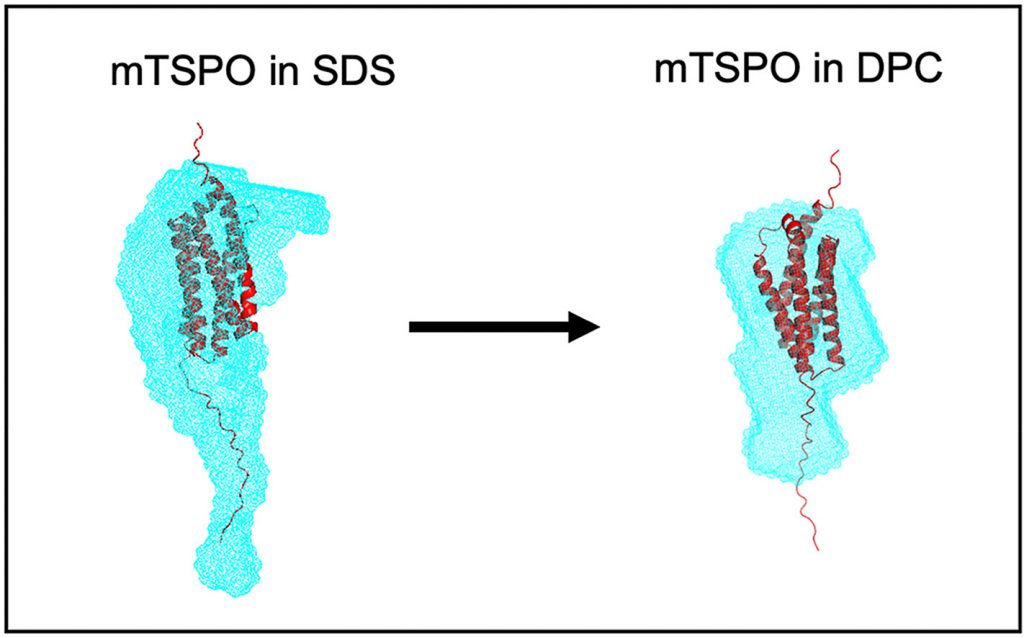
Here, we decipher the solution structures of the recombinant mTSPO without ligand both in (i) SDS, the detergent used to extract and purify the protein from E. coli inclusion bodies, and (ii) DPC, the detergent used to solve the PK 11195-binding mTSPO NMR structure.
Effect of amphiphilic environment on the solution structure
of mouse TSPO translocator protein.
We report partially refolded and less flexible mTSPO helices in DPC compared to SDS. Besides, DPC stabilizes the tertiary structure of mTSPO, as shown by a higher intrinsic Trp fluorescence and changes in indole environment.
We evaluate by SEC-MALLS that ~135 SDS and ~100 DPC molecules are bound to mTSPO. SEC-smallangle X-ray (SAXS) and neutron (SANS) scattering confirm a larger mTSPO-detergent complex in SDS than in DPC. Using the contrast-matching technique in SEC-SANS, we demonstrate that mTSPO conformation is more compact and less flexible in DPC than in SDS. Combining ab initio modeling with SANS, we confirm that mTSPO conformation is less elongated in DPC than in SDS. However, the freeligand mTSPO envelope in DPC is not as compact as the PK 11195-binding protein NMR structure, the ligand stiffening the protein.



