Short range order of a ternary Mg82Ca8Au10 biodegradable amorphous alloy was studied …
Z. Molčanová, K. Saksl, J. Ďurišin, Š. Michalik, B. Ballóková, J. Darpentigny, P. Jóvári
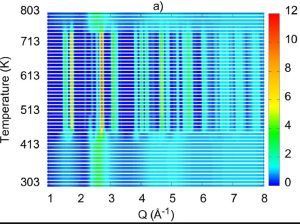
Short range order of a ternary Mg82Ca8Au10 biodegradable amorphous alloy was studied by combining diffraction datasets and Au L3 edge EXAFS data by the Reverse Monte Carlo simulation technique. It was found that while the Mg–Mg bond length agrees well with the empirical atomic diameter of Mg, both the Mg–Ca and Mg–Au mean interatomic distances are ∼9 % shorter than the sum of the corresponding atomic radii. The Ca–Au bond length exhibits ∼14 % shortening. The linear expansion coefficients of the glass determined from the temperature induced shift of the first peak of the structure factor and the reduced pair distribution function are ∼3.7 × 10−5 K−1 and ∼3.1 × 10−5 K−1, respectively. During devitrification, two crystalline phases emerge from the amorphous alloy: hexagonal AuMg3 and the solid solution of Ca in hexagonal close packed Mg. The thermal expansion behaviour of the AuMg3 unit cell was also determined using diffraction data.