In collaboration with N. Saettel, and M-P. Teulade-Fichou (Institut Curie, Orsay)
Extended planar aromatic systems represent attractive targets as active materials in electronic devices. Some of them are also commonly used in biology to achieve structure-specific recognition of DNA through p-π overlapping of nucleic bases. In order to achieve their self-organization in solution or the solid state it is therefore essential to tailor them properly. From this viewpoint, planar conjugated molecules having a threefold symmetry and bearing long alkyl chains are of particular interest because they behave as liquid crystals.
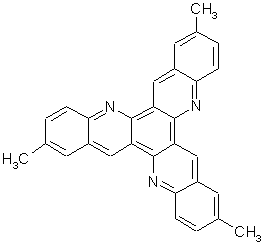
We report here on the synthesis and 2D self-assembly of 2,8,14-trimethyl-5,11,17-triazatrinaphthylene (Figure 1), the first member of a novel family of C3h-symmetry conjugated compounds noted TrisK by analogy with the celtic symbol Triskele meaning “three-legged”. The molecule of TrisK consists in a central benzene ring with three peripheral branches made of quinoline moieties, each of them terminated by a methyl group. The relative positions of the three nitrogen atoms and of the three methyl groups on the same side of each branch (clockwise and counter-clockwise respectively) make TrisK a prochiral molecule exhibiting two enantiotopic faces.
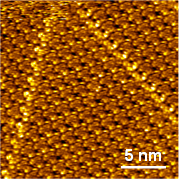
Figure 2: STM image (20.2×20.2 nm2, Ut=177 mV, It=12 pA) of a self-organized domain of TrisK at the n-tetradecane/Au(111) interface
Following the description of its chemical synthesis, we show here that compound TrisK forms long range self-assembled monolayers (SAMs) with hexagonal packing on HOPG and that high resolution STM reveals the presence of a high contrast adsorption site on one of its three branches (Figure 2). This intramolecular non-equivalent adsorption site is very surprising for a highly symmetrical molecule and is interpreted in terms of a strong local electronic interaction between certain atoms of the molecule and those of graphite. Beside, ab-initio calculations show that a purely molecular contribution may also be involved.
The large aromatic surface of TrisK makes them potentially interesting as neutral precursors of nucleic acid binders, a route which is currently under investigation in the framework of the PhD thesis of Ms. Hélène Bertrand.
References:
Triazatrinaphthylene, a three-fold symmetry planar conjugated system with two-dimensional self-assembly properties
N. Saettel, N. Katsonis, A. Marchenko, M-P. Teulade-Fichou, and D. Fichou, J. Mater. Chem. 15, 3175-3180 (2005).
• › De la molécule au matériau moléculaire  Électronique et optique du futur › Matériaux pour l'électronique et l'optique - Electronique organique et moléculaire
Électronique et optique du futur › Matériaux pour l'électronique et l'optique - Electronique organique et moléculaire
• Service de Physique et Chimie des Surfaces et des Interfaces
• Laboratory for Electronics and Photonics in Organics (LEPO) • Laboratoire d'Electronique et Photonique Organique (LEPO)