Collaboration with Collège de France, M.-P. Teulade-Fichou

Fluorescent labeling of DNA :
STM study of DNA immobilization.
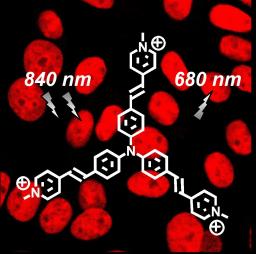
2P microscopy imaging of Chinese Hamster Ovarian cells following incubation with the trisubstituted vinylpyridinium triphenylamine derivative (TP-3py) which molecular structure is superposed (synthesis performed at College de France). The dye concentration was 2 µM. Cells were incubated at room temperature for 20 min and then washed to remove the excess of unbound dye. The slides were mounted and imaged using two-photon laser scanning microscopy with a femtosecond Ti:sapphire laser source at the maximum dye excitation wavelength (λ=840 nm). Analysis of the images showed an intense and very stable (no photodegradation) red-emission (λ =680 nm) from TP-3py which was exclusively in the nucleus indicating that TP-3py binds selectively to double stranded DNA
• Service de Physique et Chimie des Surfaces et des Interfaces • Laboratory of Physics and Chemistry of Surfaces and Interfaces
• pas de titre • Laboratoire d'Electronique et Photonique Organique (LEPO)










