Laser spectroscopies & applications
Contacts : E. Gloaguen, M. Mons
|
|
The IR / UV laser spectroscopy conducted in the gas phase (supersonic jet and laser desorption), which the group has significantly contributed to popularize, allows a thorough conformational analysis of the studied systems. Its coupling with quantum chemistry modeling enables to document with precision the intramolecular interactions that stabilize these flexible systems, in particular the intramolecular H-bonds. Thus conformational stabilization by hyperconjugation has been demonstrated (see the dedicated Highlight). The application of the IR / UV technique to systems without UV chromophores has also been documented. |
Amino-acids in the gas phase
Contacts : E. Gloaguen, M. Mons
The weakly polar side-chains of neutral amino acids such as Glycine, Alanine or Phénylalanine leads the isolated peptide chain to adopt folding conformations that correspond to its preferences within a hydrophobic environment, quite similar to that of inside a protein, such as γ-turns or β-strands.
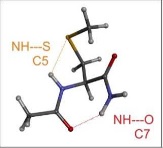
In contrast, the neutral polar amino acids, Sérine, Cystéine, Histidine or Asparagine , allow their side chain to establish specific interactions with neighboring peptide bonds, conferring these amino acids unique properties in terms of hydrogen bonding. We have characterized these H bonds in the gas phase, i.e., in the absence of solvent, and showed that they are ubiquitous in proteins, where they participate in the stabilization of secondary structures.180
Building blocks of foldamers in the gas phase
Contacts M. Mons, V. Brenner
This topic capitalizes on the team's achievements and in particular its pioneering role in the laser spectroscopy of isolated peptides. The elementary building blocks of foldamers, based on β-peptides, i.e. peptides more complex than the natural (α-) peptides, have specific intramolecular interactions, which can be characterized by IR / UV laser spectroscopy.
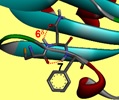
These studies are at the origin of the project ANR TUNIFOLDS, in collaboration with the groups of D. Aitken and A. Zehnacker in Orsay, which seeks to finely tune the folding properties of the building blocks, by using specific intramolecular interactions, such as NH - S hydrogen bonds, when a heteroatom sulfur is included in the building blocks. Using these specific interactions as pivots, one should be able to control the folding properties of oligomers of these β-peptides, to form various types of secondary structures (helices, ribbons, etc ...).
This project is funded by the French National Research Agency (2017-2020, ANR-17-CE29-0008)
Collaborations :
• Pr. David J. Aitken (Institut de Chimie Moléculaire et des Matériaux d'Orsay, Université Paris-Saclay)
• Anne Zehnacker, Katia LeBarbu-Debus, Valéria Lepère, (Institut des Sciences Moléculaires d'Orsay, Université Paris-Saclay)