Pages scientifiques 2007
1:
2: Laboratoire Léon Brillouin, CE-Saclay, F-91191, Gif-sur-Yvette, France
3: A. F. Ioffe Physico-Technical Institute,
4:
The properties of magnetics confined in nanometer scale cavities drastically differ from those in the bulk material. The investigation of model materials in the unusual conditions of a so-called “restricted geometry” is of fundamental interest since the confined geometry and the influence of the surface yield unusual properties.
1Groupe Matière Condensée et Matériaux, UMR-CNRS 6626, Université de Rennes 1, 35042 Rennes, France
2 Laboratoire d’Optronique, FOTON, UMR-CNRS 6082, 22302 Lannion, France
3 Laboratoire Léon Brillouin (CEA-CNRS), CEA . Saclay, 91191 Gif-sur-Yvette Cedex
Anisotropic quenched disorder effects on a liquid crystal confined into nanochannels
Intense experimental and theoretical efforts have focused on quenched disorder effects in condensed matter as they bring about some most challenging questions of modern statistical physics. Most universal features of quenched disorder effects can be envisaged in the frame of random field theories. From this standpoint, liquid-crystals (LC) confined in random porous materials are definitively recognized as paradigm systems, which allows one to address experimentally general questions on phase transitions, critical scaling and non-ergodicity in the presence of quenched random fields. (More...)
The magnetic properties (spin and orbital magnetic moments, magneto-crystalline anisotropy energy (MAE)) of nanoparticles, thin films and wires have recently attracted a lot of attention due to their potential applications mainly in the Information Technology sector.
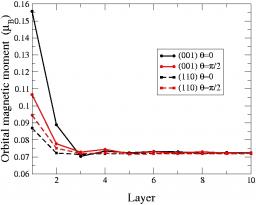
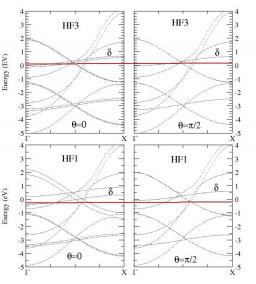
We have carried out a systematic study of the magnetic properties of iron in the tight-binding approximation including s, p, and d valence atomic orbitals taking into account spin polarisation using a Stoner-like model and in the presence of spin-orbit coupling[1]. The validity of the model has first been established by studying in details the magnetic properties of the bcc and fcc bulk phases and comparing the results to those of ab-initio calculations using the PWscf code. This model has then been applied to investigate the (110) and (001) bcc surfaces. The corresponding electronic structures and magnetic moments are very similar to those obtained from ab-initio codes. In addition we have studied the variation of the component of the orbital magnetic moment on the spin quantisation axis as a function of depth, revealing a significant enhancement in the first two layers, especially for the (001) surface (see Fig.1).
Spin electronics is an emerging science which aims at using the spin of the conduction electrons in electronic devices. In a near future, the fundamental mechanisms of spin transport will be affected by some physical limits linked to a further size reduction towards the nanometer scale. It is thus fundamental to understand these limits and more generally the physics of magnetism and transport in reduced dimensions.
Metal-metal nanocontacts are crucial in many areas, but have been poorly investigated from the point of view of local magnetism. This is a very appealing research domain, since local magnetism will greatly influence the (ballistic) conductance across a nanocontact. The break junction technique is a tool that allows the creation of stable (several hours) atomic contacts, which consists in breaking a material in a controlled manner by bending it until eventually a single atom contact appears between the two surfaces. Once a stable configuration is obtained the resistance can be measured as a function of an applied magnetic field. Michel Viret (CEA, SPEC) showed that in magnetic materials such as Fe, Co or Ni, one can obtain large magneto-resistive effects [1].
Philippe VIE (Laboratoire LMSGC, Laboratoire Central des Ponts & Chaussées, France)
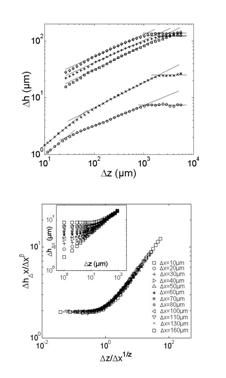
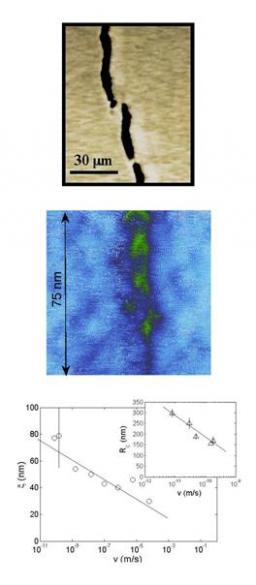
It was shown that a complete description of the statistical scaling properties of fracture surfaces in heterogeneous materials call for the use of the two-dimensional (2D) height-height correlation function /1/. This function was observed to exhibit anisotropic scaling properties similar to the Family-Viseck scaling predicted in interface growth models, characterized by three critical exponents ζ=0.75, β =0.6 and Z= ζ/β=1.2 independent to some extent of the considered material, the loading condition, and the crack growth velocity.
Laurent PONSON, Silke PRADES, Daniel BONAMY, Elisabeth BOUCHAUD, Claude GUILLOT
Cindy Lynn ROUNTREE, Silke PRADES, Daniel BONAMY, Davy DALMAS, Claude GUILLOT, Elisabeth BOUCHAUD
Coll. with Rajiv KALIA (University of Southern California, Los Angeles, USA)
Fracture of pure silica, a “minimal” elastic material has been explored both experimentally and through numerical simulations. In both cases, the crack was shown to progress through the growth and coalescence of damage cavities ahead of the front. This mechanism was shown to be responsible for small scales non linear elastic behaviour both for ultraslow stress corrosion and dynamic fracture.
1.2.1. – Fracture mechanism
Stress corrosion ultraslow (average crack velocity ranging from 1 to 100 pm/s) mode I cracks in pure amorphous silica were followed with an Atomic Force Microscope (AFM) /1/, revealing the nucleation, growth and coalescence of damage cavities (see Fig. 1).
Laurent PONSON, Daniel BONAMY, Elisabeth BOUCHAUD, Claude GUILLOT,
Coll: Harold AURADOU and Jean-Pierre HULIN (Laboratoire FAST, Université d’Orsay, France),
Guillaume MOUROT and Stéphane MOREL (Laboratoire LRBB, Université de Bordeaux, France)
Electrografting of vinylic polymers onto conducting surfaces has historically been achieved via a direct electron transfer from the cathode to the electro-active monomers in solution. The resulting radical-anion, together with the anions that allow the propagation of the growing grafted polymer chains, are highly sensitive to proton sources. The "classical" cathodic electrografting process thus requires strictly anhydrous conditions.
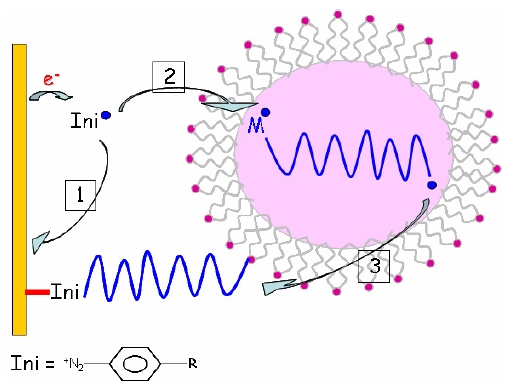
Radical polymerization does not suffer the same drawback and is easily performed in aqueous conditions. We thus demonstrated that cathodic electrografting on conducting surfaces can be achieved in protic conditions, provided the polymerization is radically initiated by an electro-active moiety. The best initiators are actually diazonium salts.
Indeed, as shown by Pinson, diazonium salts can be easily electro-grafted on conducting substrates by cathodic reduction to phenyl radical species in mild conditions. However, when the applied potential is higher than the reduction potential of the diazonium salt, a significant part of the resulting phenyl radicals are diverted to the solution and may act as initiators of radical processes. We demonstrated that this initiation is efficient and delivers polymer chains from vinylic monomers solubilized within the electrolyte (either directly or in micellar conditions). The resulting polymer chains normally grow in solution or within the micelles, and are eventually terminated by any radical or radical scavenger present in the medium. Among the termination processes, the most interesting is the reaction of the growing polymer chains with the already grafted aromatic rings coming from the direct electrografting of the diazonium initiator. Hence, the diazonium salt acts both as an initiator for the radical polymerization in solution, and as a primer for the chemical grafting of the resulting polymer chains on the surface of the electrode.
Angle-resolved XPS analysis confirmed that the polyphenylene grafted films arising from the direct electrografting of the diazonium moieties are actually located below the vinylic chains, in direct contact with the electrode.
Cathodic electropolymerization has already proven its ability to produce robust and conformal organic films chemically grafted on metallic and semiconducting surfaces used in various domains such as microelectronics, biomedical applications, soldering, chemical waste treatment or corrosion protection. Contrary to the electrodeposition of semi-conducting polymers like polypyrrole or polyaniline, strong covalent bonds between the cathode and the polymer film area created during the process, as indirectly proved by numerous studies including chemical post-treatments, electrografting on rotating electrodes or mechanical measurements. A direct evidence can however be obtained from XPS.
Probing the chemical interface between a polymer film and a metallic substrate is very difficult. Indeed, the interface is "buried" under the polymer film and thus difficult to reach. For instance, XPS cannot probe more deeply than ca. 15 nm below the outmost surface. It is thus necessary to limit the growth of the polymeric film to be able to probe the interfacial signature.
This was done by using a redox active molecule that cannot polymerize. Indeed, with classical vinylic monomers such as acrylonitrile or methyl methacrylate, the grafting step that immediately follows the electron transfer from the cathode, gives a very unstable species bearing a negative charge very close to a negatively charged electrode. The instability is classically relaxed by the polymerization that moves away the charged species from the electrode.
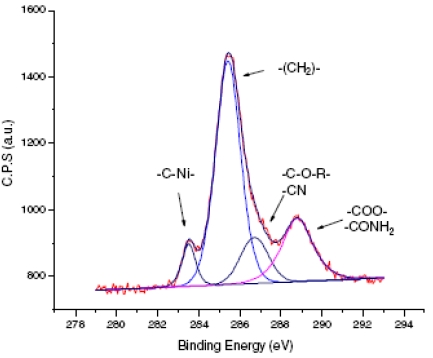
With crotononitrile, another relaxation route is possible through a proton transfer that "heals" the carbanion before polymerization occurs. Hence, ultrathin grafted films are obtained. XPS analysis of the resulting films allows the direct observation of the carbon-metal bond that is buried under the grafted film and contributes greatly to its robustness. A clear and distinct peak at 283.5 eV is observed which is attributed to C-Ni bonding. Its full width at mid-height is only 0.8 eV, which indicates that the bonding is probably perpendicular to the surface.
Contact: G. Deniau
Refrences:
[1] Carbon-to-metal bonds: Electrochemical reduction of 2-butenenitrile
Guy Deniau, Laurent Azoulay, Pascale Jégou, Gilles Le Chevallier and Serge Palacin, Surface Science, 600 (2006), 675.
[2] G. Deniau et al., Patent FR2883299, 15/03/2005
We study electro-grafted films on metal substrates as a solution for the dry lubrication of low-level electric contacts. At the moment low-level electric contacts are lubricated by means of liquids lubricants but this solution is not satisfactory for many reasons and electrical contacts still remain the weakest point of electrical circuits and a main source of failure. Among the degradation phenomena that occur in traditional liquid lubricated electrical contacts are oxidation, wear, overheating and fretting (oxidation and premature wear caused by mechanical vibrations). Many of those phenomena are caused by liquid lubricants displacement or its degradation, leading to unprotected metal-to-metal contacts.
Moreover with the increasing miniaturization of device components and high-performance materials, the surface-to-volume ratio increases, and the properties of the surfaces become increasingly more important. In many cases, especially in aerospace, high-temperature, and computer applications, it is also impractical or undesirable to have a liquid lubricant present, and one has to rely entirely on the tribological properties of dry surfaces.Therefore the functionalization of metallic surfaces with organic molecules to impart “lubricating” properties becomes a subject of major interest both from fundamental and technological points of view.
With respect to other techniques for grafting organic molecules on metal surfaces (SAM, Langmuir-Blodgett films, …) electrografting allows the formation of strong covalent bonds at the organic/inorganic interface leading to the formation of highly-adherent thin-films (thickness < 50nm). The electrografting of fluorinated molecules (fluorinated diazonium salts or fluorinated methacrylates) allows obtaining robust films providing low-friction properties.
As those films are naturally insulating, the necessary electrical transport through the metal-organic-metal interface can be borne by adding a conducting or semi conducting charge to the films. In particular we concentrate on carbon nanotubes networks and on conducting fluorinated PEDOT.
Our early results on grafted fluorinated polymethacrylates films show excellent behaviour in term of stability and tribological properties, reaching friction coefficient as low as 0.1 and showing wear resistance in severe testing conditions. (Fig. 1).
The localized grafting, at a micronic or submicronic scale, of organic substances on surfaces is often a prerequisite in the design of bioelectronics devices, and a valuable component of some combinatorial screening strategies. Moreover, microelectronic devices including transistors, sensors or memories are generally based on interfaces between conducting and quasi-insulating domains resulting from lithographic steps, ionic implantation and silicon oxide thermal growth. Most of these techniques require many steps of transformation and their implementation is often expensive.
We developed a one step technique that allows the localized electrografting of organic matter on designed areas of a composite conducting surface. This method relies on the local work function of electrons, which can easily be tuned by patterning different conducting materials on the substrate. [1-2]
The method was originally demonstrated through examples of composite gold/silicon substrates, which underwent organic electrografting only on gold parts, even when the potential was applied via the silicon substrate and the gold parts were geometrically isolated from the voltage source. That effect is due to:
• The mechanism of cathodic electrografting, which is initiated by an adsorbed radical-anion resulting from a direct electron-transfer from the cathode to electro-active molecules
• The weaker work function of gold with respect to silicon (in solution), which leads to more efficient "output" kinetics from the golden areas.
Electrografting is a powerful technique that allows true chemical modification of conducting and semi-conducting surfaces. [1] Indeed, injecting electrons from the bulk material towards a solution of suitable electroactive molecules leads to the formation of activated moieties in the vicinity of the electrode, which eventually form strong chemical links with the electrode.
Our group has historically been developing the electrografting of vinylic polymers via an electro-induced anionic polymerization that starts from a precursor chemically bound to the electrode.
Strong efforts were devoted in past years both to the theoretical interpretation of the mechanism and towards practical applications of the process. That latter approach was rewarded in 2001 by the creation of a spin-off company, Alchimer, which applies the process in the biomedicine and microelectronics fields. [2-13]
From that moment, our group carried on support activities to develop and enrich the electrografting process. We first demonstrated that biological moieties can be directly immobilized on electrografted polymethacrylonitrile films via a spontaneous reaction between the nitrile groups from the film and the amino groups from the amino acids of the protein, in alcoholic basic medium. The formation of the resulting imino-ethers groups was followed by XPS measurements and assessed by high precision calculations of core electron binding energies. The final protein film is stable toward aqueous rinsing and can be used for protein-substrate recognition. [14]