•  Physique et chimie pour le vivant et l’environnement › Physique et vivant / Physics and life
Physique et chimie pour le vivant et l’environnement › Physique et vivant / Physics and life  Physics and chemistry for life sciences and the environment
Physics and chemistry for life sciences and the environment
• Laboratoire Léon Brillouin (LLB) • Leon Brillouin Laboratory (LLB)
• Groupe "Matière molle et biophysique" - MMB • Small angle scattering
• Neutrons • Activités instrumentales nationales et internationales du LLB - National and internatonial instrumental activities at the LLB • Les appareils d'Orphée; Orphée neutrons spectrometers • Neutrons • Small Angle Neutron Scattering (PA)



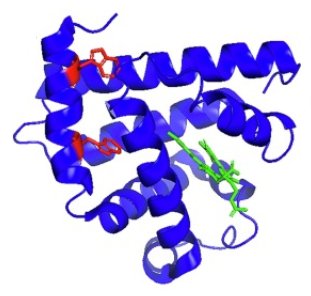
Ribbon model of the crystal structure of apoMb (PDB:1BVC) in complex with BV. Tryptophan (Trp) aromatic side chains and BV are shown in red (Trp7 and Trp14) and green colours, respectively;
Simeon Minic, Burkhard Annighöfer, Milos Milcic, François Maignen, Annie Brûlet, Sophie Combet
Apomyoglobin (apoMb), a model protein in biochemistry, exhibits a strong propensity to bind various ligands, which makes it a good candidate as a carrier of bioactive hydrophobic drugs. The stability of its hydrophobic pocket determines its potential as a carrier of bioactive compounds. High pressure (HP) is a potent tool for studying protein stability, revealing the specific role of hydrophobic cavities in unfolding. We probed the effects of biliverdin (BV) binding and its complex with Zn2+ ions on the structure and HP stability of apoMb. CD spectroscopy and SAXS measurements revealed that BV and BV-Zn2+ complexes make the apoMb structure more compact with higher α-helical content. We performed in situ HP measurements of apoMb intrinsic fluorescence to demonstrate the ability of BV to stabilise apoMb structure at HP conditions. Furthermore, the presence of Zn2+ within the apoMb-BV complex significantly enhances the BV stabilisation effect. In situ visible absorption study of BV chromophore confirmed the ability of Zn2+ to increase the stability of apoMb-BV complex under HP: the onset of complex dissociation is shifted by ∼100 MPa in presence of Zn2+. By combining HP-fluorescence and HP-visible absorption spectroscopy, our strategy highlights the crucial role of tetrapyrrole-metal complexes in stabilising apoMb hydrophobic pocket.


