At a time when we question the fossil fuel reserves of our planet and the consequences of the greenhouse effect on global warming, hydrogen is seen as the future energy vector for transportation. Research within CEA cover all stages of this chain: production, storage, transport, distribution and use. In that field, hydrogen produced from primary energy, solar, nuclear, wind, chemical ... is stored in the tank of a vehicle and a fuel cell, allowing the clean conversion (without CO2 emission) of chemical energy into electrical energy, combined with an electric engine can replace the gasoline engine of our cars.
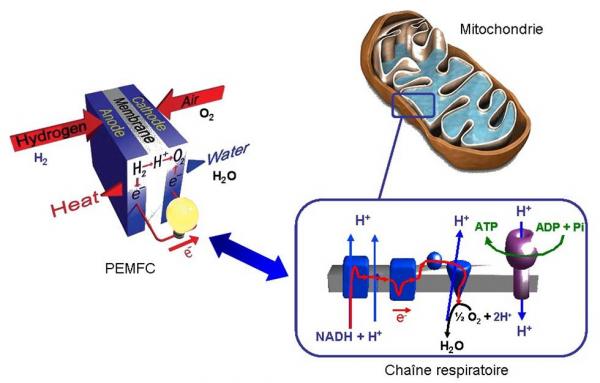
Among the various types of fuel cells suitable for transport applications, the most interesting are of PEMFC type (Proton Exchange Membrane Fuel Cell). These fuel cells contain, in particular, a polymer playing the role of the solid electrolyte. The Dupont De Nemours company sells a sulfonated perfluorocarbon membrane named Nafion®. However, this membrane presents some drawbacks as a mediocre autonomy (< 5000h operating), a mechanical weakness or the inability to function in anhydrous media… The "Irradiated Polymer" research group within LSI is trying to address these problems by proposing a new type of membrane.
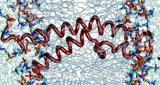
The original approach is to mimic proteins in some cellular organelles such as mitochondria. Indeed, an analogy can be drawn between a PEMFC and the mitochondria which are "the fuel cells" of eucaryotic cells.
In a PEMFC, hydrogen from the anode is dissociated into protons and electrons following the oxidation reaction: H2 → 2 H+ + 2 e-. Thus, a strong difference in proton concentration between the anode and the cathode follows, that generates an electrochemical force that is accentuated by the 2H+ + 2e- + 1/2 O2 → H2O recombination. This is very similar to what happens in the respiratory chain within a mitochondria: an electrochemical gradient of protons is generated on both sides of the mitochondrial membrane ([H+]ext> [H+]int).
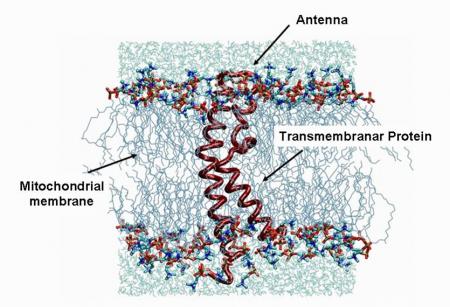
This electrochemical force contributes to generate energy in the form of ATP (Adenosine TriPhosphate) at the level of a subunit of a transmembrane protein (equivalent to one channel): the ATP synthase. The structural study (use and spatial distribution of the various functions for proton conduction) of this protein helps to understand the very good performance of the proton transfer.
It is therefore interesting to mimic this type of protein as well as its environment in a perfluorinated membrane. The film medium is a thermoplastic polymer type (PVDF) irradiated with swift heavy ions at GANIL and radiografted by a polyelectrolyte along the tracks of the ion pathways (Figure 3).
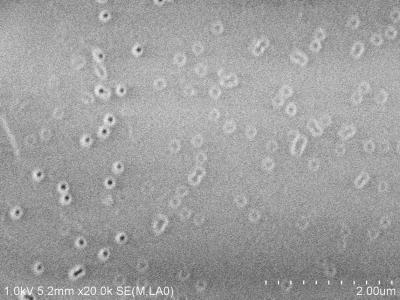
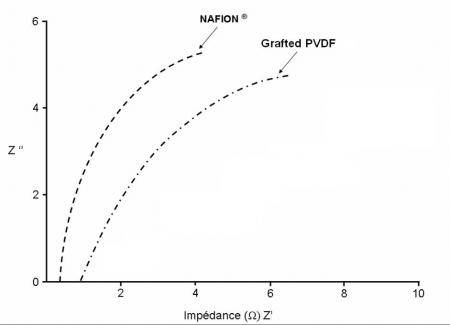
Nanochannels for proton circulation are thus created from the anode to the cathode. By its good mechanical properties at low and high temperature for a thickness of ~ 10 μm and by its impermeability to gases in fuel cell operating condition, this type of membrane would be an excellent product for PEMFC. The functionalization of the polyelectrolyte within the conduction channels with various proton conductive entities allowed us to validate the approach. Other developments are underway to make it more competitive in terms of conductivity (Figure 4).
Reference:
FR 07 57873 and FR 07 57875 (patents deposited september 2007)
* LSI : Laboratoire des Solides irradiés, joint laboratory CEA (DSM/IRAMIS), CNRS (UMR 7642) and Ecole Polytechnique.
•  Les archives de l'IRAMIS et du DRECAM / Archives of DRECAM and IRAMIS › Surfaces and nanostructures
Les archives de l'IRAMIS et du DRECAM / Archives of DRECAM and IRAMIS › Surfaces and nanostructures