Laboratoire Léon Brillouin
UMR12 CEA-CNRS, Bât. 563 CEA Saclay
91191 Gif sur Yvette Cedex, France
+33-169085241 llb-sec@cea.fr
Ecotoxicological impacts of Nanoparticles
Thanks to their particular or enhanced properties due to their size, the nanomaterials (dimension < 100 nm) are widely used in many industrial applications (daily care products, nanostructured materials...). However, their growing use together with the belief of easy dispersability frightens because of their uncertain impact on humans and environment. Our work is dedicated to a deep understanding of the physicochemical and biological interactions between cellular models such as: Synechocystis (cyanobacteria essential for the biosphere) or Escherichia coli (bacteria of mammalian intestines) with nanoparticles (CeO2, TiO2, Ag0, Au).
The particular behavior of nanoparticles (agregation, dissolution, adsorption...) requires a different and highly multidisciplinary approach compared to the study involving classic chemical compounds. Indeed, we showed that the physicochemical parameters (stability, aggregation, dissolution and surface chemistry) of nanoparticles in the contact medium, strongly influence the toxicity. Furthermore, the physicochemical interactions (flocculation, adsorption, redox mechanisms) are linked to the biological model; for exemple, the presence of exopolysaccharides as natural barrier between the cell wall and the nanoparticles. Moreover, the composition of the nanoparticles dispersion medium (particularly the pH) has a major influence on the toxicity.
Many of these research activities are carried in the framework of the international Consortium for the Environemental Impact of Nanotechnologies (iCEINT). The LIONS is also leading a CNano Project of the region Ile de France, regarding the interaction between nanoparticles and bacteria in the Seine River. The project is at the frontier between physics and chemistry of NPs, biology and ecotoxicology.
The inorganic manufactured nanoparticles as TiO2, Ag°, the iron oxides and CeO2 are more and more present in various manufactured products and in the aqueous media (TiO2). Their dispersion in the ecosystems during their life cycle will be associated with interactions with biota (plants, bacteria, fishes). The present work shows strong relations between particular physical chemical properties of very small nanoparticles (size < 30 nm) and biological activity perturbations. It is shown that Ag° and CeO2 act at very low concentrations. TiO2 act via the ROS production due to their photo-reactivity.

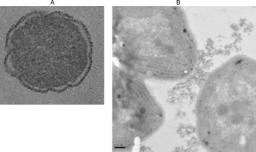
Left : SEM picture of Synechocystis after critical CO2 drying. right : TEM section of E. coli after contact with CeO2. the inset show detail of the cell wall.
Interaction of NPs with bacteria : effect of polysaccharides
Physico-chemical interactions between nanoparticles and cell membranes play a crucial role in determining the cytotoxicity of nanoparticles, which may thereby vary depending on the nature of the target microorganisms. We investigated the responses of two different models of unicellular bacteria to cerium oxide (CeO2) nanoparticles. These organisms are: Synechocystis PCC6803 a representative of environmentally important cyanobacterial organisms (producer of biomass for aquatic food chains), and Escherichia coli a representative of intestine-colonizing bacteria. Coupling physico-chemical (adsorption isotherms and electrophoretic mobility), biological (survival tests), microscopical (SEM, TEM and EDS) and spectroscopic (XANES) methods, we enlightened two distinct mechanisms for the CeO2 nanoparticles toxicological impact: A ‘direct’ mechanism that requires a close contact between nanoparticles and cell membranes, and an ‘indirect’ influence elicited by the acidity of nanoparticles stabilizing agents. We showed that E. coli is sensitive to the ‘direct’ effects of nanoparticles, whereas Synechocystis being protected by extracellular polymeric substances preventing direct cellular contacts is sensitive only to the ‘indirect’ mechanism. Consequently, our findings demonstrate the importance of the ‘direct/indirect’ effects of nanoparticles on cell fitness, a phenomenon that should be systematically investigated with appropriate techniques and dose metrics to make meaningful environmental and/or health recommendations.
Genotoxicity of cerium dioxide
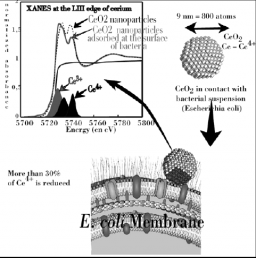
Cerium dioxide nanoparticles have been proposed for an increasing number of applications in biomedicine, cosmetic, as polishing materials and also as byproducts from automotive fuel additives. The aim of this study was to examine the potential in vitro cyto- and genotoxicity of nano-sized CeO2 (7 nm) on human dermal fibroblasts. By combining a physico-chemical and a (geno)toxicological approach, we defined the causal mechanisms linking the physico-chemical properties of nano-CeO2 with their biological effects. Using X-ray absorption spectroscopy, we observed a reduction of 21±4% of the Ce4+ atoms localized at the surface of CeO2 nanoparticles due to the interactions with organic molecules present in biological media. These particles induced strong DNA lesions and chromosome damage related to an oxidative stress. These genotoxic effects occurred at very low doses, which highlighted the importance of a genotoxicological approach during the assessment of the toxicity of nanoparticles.
Learn more...
Nanogenotoxicity
The LIONS group is also participating to a European Joint Action "NANOGENOTOX" regarding the relation between in vivo and in vitro tests. We have developed special protocols of NPs dispersion in complex cell culture media.
Cytotoxicity of cerium dioxide for E. coli
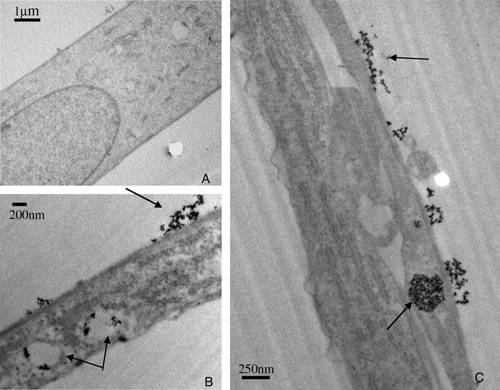
Biological activity is playing a crucial role in the cycling of elements at the Earth’s surface. With the development of human activity new solid materials are dispersed thereby raising the question of their mobility, toxicity and persistence in the ecosystems. This is particularly true concerning the development of nanomaterials. The general context of our work is to assess the role of micro-organisms in the life cycle of manufactured nanomaterials (toxicity, biodegradation…) More specifically we aim at determining the mechanisms of interaction between manufactured nanomaterials and cells. Cerium and iron oxides were used in contact with E. coli cells. Results combining various microscopic (TEM) and synchrotron based spectroscopic techniques (XAS) indicated that redox phenomena occurred at the cell wall surface and can be linked to toxicological effects. Such redox phenomena also affected the stability of manufactured nanomaterials.
In this work, we have studied the affinity of pure C60 fullerenes with water. Although C60 have always been known as hydrophobic particles, we show that this characteristic does not remain when contact between C60 and H2O is forced by mechanical stirring. Light scattering measurements and transmission electron microscopy observations revealed the presence of C60 aggregates remaining stable in water after stirring, with a large nanometric size range. Water adsorption/desorption isotherms, confirmed the hydrophilic character of these nano‐clusters, resulting form hydration of the initial hydrophobic C60.
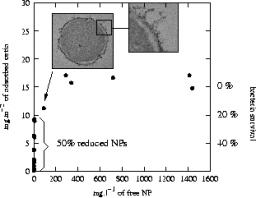
The production of nanoparticles (NPs) is increasing rapidly for applications in electronics, chemistry, and biology. This interest is due to the very small size of NPs which provides them with many interesting properties such as rapid diffusion, high specific surface areas, reactivity in liquid or gas phase, and a size close to biomacromolecules. In turn, these extreme abilities might be a problem when considering a potentially uncontrolled exposure to the environment. For instance, nanoparticles might be highly mobile and rapidly transported in the environment or inside the body through a water or air pathway. Accordingly, the very fast development of these new synthetic nanomaterials raises questions about their impact on the environment and human health. We have studied the impact of a model water dispersion of nanoparticles (7 nm CeO2 oxide) on a Gram-negative bacteria (Escherichia coli). The nanoparticles are positively charged at neutral pH and thus display a strong electrostatic attraction toward bacterial outer membranes. The counting of colony forming units (CFU) after direct contact with CeO2 NPs allows for the defining of the conditions for which the contact is lethal to Escherichia coli. Furthermore, a set of experiments including sorption isotherms, TEM microscopy, and X-ray absorption spectroscopy (XAS) at cerium L3 edge is linked to propose a scenario for the observed toxic contact.
• › Nanostructures et biomolécules : biomédecine et nanotoxicité / Nanostructures and biomolecules: biomedicine and nanotoxicity  Physique et chimie pour le vivant et l’environnement
Physique et chimie pour le vivant et l’environnement  Synthèse et caractérisation des nano-objets / Synthesis and characterization of nano-objects
Synthèse et caractérisation des nano-objets / Synthesis and characterization of nano-objects
• UMR 3299 - Service Interdisciplinaire sur les Systèmes Moléculaires et les Matériaux • Service Interdisciplinaire sur les Systèmes Moléculaires et les Matériaux
• Laboratoire Interdisciplinaire sur l'Organisation Nanométrique et Supramoléculaire (LIONS) • Interdisciplinary Laboratory on Nanoscale and Supramolecular Organization